Metabolic Coordination of Physiological and Pathological Cardiac Remodeling
- PMID: 29929976
- PMCID: PMC6023588
- DOI: 10.1161/CIRCRESAHA.118.312017
Metabolic Coordination of Physiological and Pathological Cardiac Remodeling
Abstract
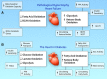
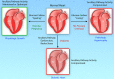
Metabolic pathways integrate to support tissue homeostasis and to prompt changes in cell phenotype. In particular, the heart consumes relatively large amounts of substrate not only to regenerate ATP for contraction but also to sustain biosynthetic reactions for replacement of cellular building blocks. Metabolic pathways also control intracellular redox state, and metabolic intermediates and end products provide signals that prompt changes in enzymatic activity and gene expression. Mounting evidence suggests that the changes in cardiac metabolism that occur during development, exercise, and pregnancy as well as with pathological stress (eg, myocardial infarction, pressure overload) are causative in cardiac remodeling. Metabolism-mediated changes in gene expression, metabolite signaling, and the channeling of glucose-derived carbon toward anabolic pathways seem critical for physiological growth of the heart, and metabolic inefficiency and loss of coordinated anabolic activity are emerging as proximal causes of pathological remodeling. This review integrates knowledge of different forms of cardiac remodeling to develop general models of how relationships between catabolic and anabolic glucose metabolism may fortify cardiac health or promote (mal)adaptive myocardial remodeling. Adoption of conceptual frameworks based in relational biology may enable further understanding of how metabolism regulates cardiac structure and function.
Keywords: exercise; glucose; heart failure; hypertrophy; mitochondria; pregnancy; systems biology.
© 2018 The Authors.
Figures

References
-
- Taegtmeyer H, Lam T, Davogustto G. Cardiac metabolism in perspective. Compr Physiol. 2016;6:1675–1699. doi: 10.1002/cphy.c150056. - PubMed
-
- Opie LH. Metabolism of the heart in health and disease. II. Am Heart J. 1969;77:100–122 contd. - PubMed
-
- Opie LH. Heart Physiology: From Cell to Circulation. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 308–354.
-
- Schönekess BO. Competition between lactate and fatty acids as sources of ATP in the isolated working rat heart. J Mol Cell Cardiol. 1997;29:2725–2733. doi: 10.1006/jmcc.1997.0504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

