A20 and ABIN-1 synergistically preserve intestinal epithelial cell survival
- PMID: 29930103
- PMCID: PMC6028510
- DOI: 10.1084/jem.20180198
A20 and ABIN-1 synergistically preserve intestinal epithelial cell survival
Abstract
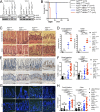
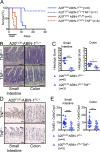
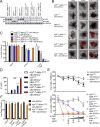
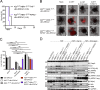
A20 (TNFAIP3) and ABIN-1 (TNIP1) are candidate susceptibility genes for inflammatory bowel disease and other autoimmune or inflammatory diseases, but it is unclear how these proteins interact in vivo to prevent disease. Here we show that intestinal epithelial cell (IEC)-specific deletion of either A20 or ABIN-1 alone leads to negligible IEC loss, whereas simultaneous deletion of both A20 and ABIN-1 leads to rapid IEC death and mouse lethality. Deletion of both A20 and ABIN-1 from enteroids causes spontaneous cell death in the absence of microbes or hematopoietic cells. Studies with enteroids reveal that A20 and ABIN-1 synergistically restrict death by inhibiting TNF-induced caspase 8 activation and RIPK1 kinase activity. Inhibition of RIPK1 kinase activity alone, or caspase inhibition combined with RIPK3 deletion, abrogates IEC death by blocking both apoptosis and necroptosis in A20 and ABIN-1 double-deficient cells. These data show that the disease susceptibility proteins A20 and ABIN-1 synergistically prevent intestinal inflammation by restricting IEC death and preserving tissue integrity.
© 2018 Kattah et al.
Figures

Comment in
-
A20 and ABIN-1 team up against intestinal epithelial cell death.J Exp Med. 2018 Jul 2;215(7):1771-1773. doi: 10.1084/jem.20180953. Epub 2018 Jun 22. J Exp Med. 2018. PMID: 29934322 Free PMC article.
References
-
- Berger S.B., Harris P., Nagilla R., Kasparcova V., Hoffman S., Swift B., Dare L., Schaeffer M., Capriotti C., Ouellette M., et al. . 2015. Characterization of GSK’963: A structurally distinct, potent and selective inhibitor of RIP1 kinase. Cell Death Discov. 1:15009 10.1038/cddiscovery.2015.9 - DOI - PMC - PubMed
-
- Bruno M.E.C., Rogier E.W., Arsenescu R.I., Flomenhoft D.R., Kurkjian C.J., Ellis G.I., and Kaetzel C.S.. 2015. Correlation of biomarker expression in colonic mucosa with disease phenotype in Crohn’s disease and ulcerative colitis. Dig. Dis. Sci. 60:2976–2984. 10.1007/s10620-015-3700-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

