Cdk1 phosphorylation of the dynein adapter Nde1 controls cargo binding from G2 to anaphase
- PMID: 29930206
- PMCID: PMC6122996
- DOI: 10.1083/jcb.201707081
Cdk1 phosphorylation of the dynein adapter Nde1 controls cargo binding from G2 to anaphase
Abstract
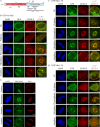
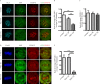
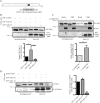
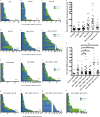
Cytoplasmic dynein is involved in diverse cell cycle-dependent functions regulated by several accessory factors, including Nde1 and Ndel1. Little is known about the role of these proteins in dynein cargo binding, and less is known about their cell cycle--dependent dynein regulation. Using Nde1 RNAi, mutant cDNAs, and a phosphorylation site-specific antibody, we found a specific association of phospho-Nde1 with the late G2-M nuclear envelope and prophase to anaphase kinetochores, comparable to the pattern for the Nde1 interactor CENP-F. Phosphomutant-Nde1 associated only with prometaphase kinetochores and showed weaker CENP-F binding in in vitro assays. Nde1 RNAi caused severe delays in mitotic progression, which were substantially rescued by both phosphomimetic and phosphomutant Nde1. Expression of a dynein-binding-deficient Nde1 mutant reduced kinetochore dynein by half, indicating a major role for Nde1 in kinetochore dynein recruitment. These results establish CENP-F as the first well-characterized Nde1 cargo protein, and reveal phosphorylation control of Nde1 cargo binding throughout a substantial fraction of the cell cycle.
© 2018 Wynne and Vallee.
Figures
Similar articles
-
Cenp-F links kinetochores to Ndel1/Nde1/Lis1/dynein microtubule motor complexes.Curr Biol. 2007 Jul 3;17(13):1173-9. doi: 10.1016/j.cub.2007.05.077. Curr Biol. 2007. PMID: 17600710
-
Phosphorylation and Pin1 binding to the LIC1 subunit selectively regulate mitotic dynein functions.J Cell Biol. 2021 Dec 6;220(12):e202005184. doi: 10.1083/jcb.202005184. Epub 2021 Oct 28. J Cell Biol. 2021. PMID: 34709360 Free PMC article.
-
Nudel modulates kinetochore association and function of cytoplasmic dynein in M phase.Mol Biol Cell. 2007 Jul;18(7):2656-66. doi: 10.1091/mbc.e06-04-0345. Epub 2007 May 9. Mol Biol Cell. 2007. PMID: 17494871 Free PMC article.
-
Dynein at the kinetochore.J Cell Sci. 2023 Mar 1;136(5):jcs220269. doi: 10.1242/jcs.220269. Epub 2023 Mar 2. J Cell Sci. 2023. PMID: 36861883 Review.
-
Chromosome movement: dynein-out at the kinetochore.Curr Biol. 2001 Feb 20;11(4):R128-31. doi: 10.1016/s0960-9822(01)00059-8. Curr Biol. 2001. PMID: 11250166 Review.
Cited by
-
Zombies Never Die: The Double Life Bub1 Lives in Mitosis.Front Cell Dev Biol. 2022 May 13;10:870745. doi: 10.3389/fcell.2022.870745. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35646932 Free PMC article. Review.
-
Reversible and effective cell cycle synchronization method for studying stage-specific processes.Life Sci Alliance. 2025 Mar 4;8(5):e202403000. doi: 10.26508/lsa.202403000. Print 2025 May. Life Sci Alliance. 2025. PMID: 40037894 Free PMC article.
-
Ndel1 modulates dynein activation in two distinct ways.bioRxiv [Preprint]. 2023 Jan 25:2023.01.25.525437. doi: 10.1101/2023.01.25.525437. bioRxiv. 2023. Update in: J Biol Chem. 2023 Jun;299(6):104735. doi: 10.1016/j.jbc.2023.104735. PMID: 36747695 Free PMC article. Updated. Preprint.
-
Nde1 and Ndel1: Outstanding Mysteries in Dynein-Mediated Transport.Front Cell Dev Biol. 2022 Apr 12;10:871935. doi: 10.3389/fcell.2022.871935. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35493069 Free PMC article. Review.
-
Nesprin-2 Recruitment of BicD2 to the Nuclear Envelope Controls Dynein/Kinesin-Mediated Neuronal Migration In Vivo.Curr Biol. 2020 Aug 17;30(16):3116-3129.e4. doi: 10.1016/j.cub.2020.05.091. Epub 2020 Jul 2. Curr Biol. 2020. PMID: 32619477 Free PMC article.
References
-
- Alkuraya F.S., Cai X., Emery C., Mochida G.H., Al-Dosari M.S., Felie J.M., Hill R.S., Barry B.J., Partlow J.N., Gascon G.G., et al. . 2011. Human mutations in NDE1 cause extreme microcephaly with lissencephaly [corrected]. Am. J. Hum. Genet. 88:536–547. 10.1016/j.ajhg.2011.04.003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

