A widely-applicable high-throughput cellular thermal shift assay (CETSA) using split Nano Luciferase
- PMID: 29930256
- PMCID: PMC6013488
- DOI: 10.1038/s41598-018-27834-y
A widely-applicable high-throughput cellular thermal shift assay (CETSA) using split Nano Luciferase
Abstract
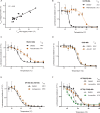
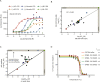
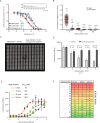
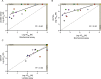
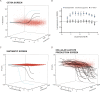
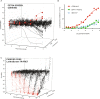
Assessment of the interactions between a drug and its protein target in a physiologically relevant cellular environment constitutes a major challenge in the pre-clinical drug discovery space. The Cellular Thermal Shift Assay (CETSA) enables such an assessment by quantifying the changes in the thermal stability of proteins upon ligand binding in intact cells. Here, we present the development and validation of a homogeneous, standardized, target-independent, and high-throughput (384- and 1536-well formats) CETSA platform that uses a split Nano Luciferase approach (SplitLuc CETSA). The broad applicability of the assay was demonstrated for diverse targets, and its performance was compared with independent biochemical and cell-based readouts using a set of well-characterized inhibitors. Moreover, we investigated the utility of the platform as a primary assay for high-throughput screening. The SplitLuc CETSA presented here enables target engagement studies for medium and high-throughput applications. Additionally, it provides a rapid assay development and screening platform for targets where phenotypic or other cell-based assays are not readily available.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Auld, D. S. et al. Receptor Binding Assays for HTS and Drug Discovery, in Assay Guidance Manual. (eds G. S. Sittampalam et al.) (Bethesda (MD); 2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

