Endothelial Mechanotransduction, Redox Signaling and the Regulation of Vascular Inflammatory Pathways
- PMID: 29930512
- PMCID: PMC5999754
- DOI: 10.3389/fphys.2018.00524
Endothelial Mechanotransduction, Redox Signaling and the Regulation of Vascular Inflammatory Pathways
Abstract
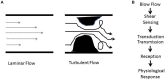
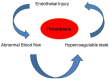
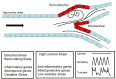
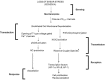
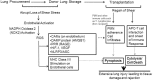
The endothelium that lines the interior of blood vessels is directly exposed to blood flow. The shear stress arising from blood flow is "sensed" by the endothelium and is "transduced" into biochemical signals that eventually control vascular tone and homeostasis. Sensing and transduction of physical forces occur via signaling processes whereby the forces associated with blood flow are "sensed" by a mechanotransduction machinery comprising of several endothelial cell elements. Endothelial "sensing" involves converting the physical cues into cellular signaling events such as altered membrane potential and activation of kinases, which are "transmission" signals that cause oxidant production. Oxidants produced are the "transducers" of the mechanical signals? What is the function of these oxidants/redox signals? Extensive data from various studies indicate that redox signals initiate inflammation signaling pathways which in turn can compromise vascular health. Thus, inflammation, a major response to infection or endotoxins, can also be initiated by the endothelium in response to various flow patterns ranging from aberrant flow to alteration of flow such as cessation or sudden increase in blood flow. Indeed, our work has shown that endothelial mechanotransduction signaling pathways participate in generation of redox signals that affect the oxidant and inflammation status of cells. Our goal in this review article is to summarize the endothelial mechanotransduction pathways that are activated with stop of blood flow and with aberrant flow patterns; in doing so we focus on the complex link between mechanical forces and inflammation on the endothelium. Since this "inflammation susceptible" phenotype is emerging as a trigger for pathologies ranging from atherosclerosis to rejection post-organ transplant, an understanding of the endothelial machinery that triggers these processes is very crucial and timely.
Keywords: endothelial mechanotransduction; inflammation; redox signals; revascularization; vascular disease.
Figures

References
-
- Alberts-Grill N., Rezvan A., Son D. J., Qiu H., Kim C. W., Kemp M. L., et al. . (2012). Dynamic immune cell accumulation during flow-induced atherogenesis in mouse carotid artery: an expanded flow cytometry method. Arterioscler. Thromb. Vasc. Biol. 32, 623–632. 10.1161/ATVBAHA.111.242180 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

