Topical vs. intravenous administration of tranexamic acid in knee arthroplasty and prevalence of deep venous thrombosis: a randomized clinical trial
- PMID: 29930576
- PMCID: PMC5829705
- DOI: 10.1590/1677-5449.007515
Topical vs. intravenous administration of tranexamic acid in knee arthroplasty and prevalence of deep venous thrombosis: a randomized clinical trial
Abstract
Background: Tranexamic acid (TXA) is widely used in orthopedic surgery to reduce perioperative bleeding. Since TXA inhibits fibrinolysis, there is concern that it may increase the risk of thromboembolic events.
Objectives: To verify the prevalence of deep venous thrombosis (DVT) in patients receiving TXA during total knee arthroplasty and to compare topical with intravenous administration of the drug.
Methods: All patients admitted for total knee arthroplasty due to primary arthrosis between June and November of 2014 were recruited consecutively. Thirty patients were randomized to a "topical group" (1.5 g TXA diluted in 50ml saline sprayed over the area operated, before tourniquet release), 30 to an "intravenous group" (20mg/kg TXA in 100 ml of saline, given at the same time as anesthesia), and 30 to a control group (100 ml of saline, given at the same time as anesthesia). All patients had duplex ultrasound scans of the legs on the 15th postoperative day.
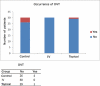
Results: Deep venous thrombosis events occurred in five of the 90 patients operated (one out of 30 in the topical group [3.3%], four out of 30 in the control group [13.3%], and zero in the intravenous group). All were confirmed by duplex ultrasound scans and all were asymptomatic. Prevalence rates of DVT were similar between groups (p = 0.112 for control vs. intravenous; p = 0.353 for control vs. topical; and p =1.000 for intravenous vs. topical, according to two-sided exact tests).
Conclusions: Both topical and intravenous administration of TXA are safe with regard to occurrence of DVT, since the number of DVT cases in patients given TXA was not different to the number in those given placebo.
Contexto: O ácido tranexâmico é amplamente utilizado em cirurgia ortopédica para reduzir a hemorragia perioperatória. Como o ácido tranexâmico inibe a fibrinólise, há uma preocupação de que ele possa aumentar o risco de eventos tromboembólicos.
Objetivos: Verificar se o uso do ácido tranexâmico é seguro em relação à prevalência de trombose venosa profunda em pacientes submetidos a artroplastia total do joelho, e comparar as administrações tópica e intravenosa desse medicamento.
Métodos: Todos os pacientes consecutivamente admitidos para artroplastia total do joelho devido a artrose primária entre junho e novembro de 2014 foram recrutados. Os pacientes foram randomizados em um “grupo tópico” (1,5 g de ácido tranexâmico diluído em 50 ml de solução salina cobrindo toda a área operada antes de liberar o torniquete), um “grupo intravenoso” (20 mg/kg de ácido tranexâmico em 100 ml de solução salina no momento da anestesia) e um “grupo controle” (100 ml de solução salina com a anestesia). No 15º dia de pós-operatório, todos os pacientes foram submetidos a ultrassonografia vascular com Doppler de membros inferiores, independentemente de sintomas.
Resultados: Dos 90 pacientes operados, apenas cinco apresentaram trombose venosa profunda (um no grupo tópico e quatro no grupo controle).
Conclusões: Tanto a administração tópica quanto a intravenosa de ácido tranexâmico são seguras em termos de ocorrência de trombose venosa profunda, pois o número de casos de trombose venosa profunda foi semelhante quando comparamos os pacientes que receberam ácido tranexâmico e os que receberam placebo. Novos estudos, com amostras maiores, são necessários para confirmar esse achado.
Keywords: antifibrinolytic agents; arthroplasty; fibrin modulating agents; knee replacement; tranexamic acid; venous thrombosis.
Conflict of interest statement
Conflicts of interest: No conflicts of interest declared concerning the publication of this article.
Figures
References
-
- Leme LE, Sguizzatto GT. Prophylaxis of venous thromboembolism in orthopaedic surgery. Rev Bras Ortop. 2012;47(6):685–693. http://dx.doi.org/10.1590/S0102-36162012000600002 - DOI - PMC - PubMed
-
- Menzin J, Richner R, Huse D, Colditz GA, Oster G. Prevention of deep-vein thrombosis following total hip replacement surgery with enoxaparin versus unfractionated heparin: a pharmacoeconomic evaluation. Ann Pharmacother. 1994;28(2):271–275. - PubMed
-
- Bredbacka S, Andreen M, Blombäck M, Wykman A. Activation of cascade systems by hip arthroplasty. No difference between fixation with and without cement. Acta Orthop Scand. 1987;58(3):231–235. http://dx.doi.org/10.3109/17453678709146472 - DOI - PubMed
-
- Eriksson BI, Eriksson E, Gyzander E, Teger-Nilsson AC, Risberg B. Thrombosis after hip replacement. Relationship to the fibrinolytic system. Acta Orthop Scand. 1989;60(2):159–163. http://dx.doi.org/10.3109/17453678909149244 - DOI - PubMed
-
- Francis CW, Ricotta JJ, Evarts CM, Marder VJ. Long-term clinical observations and venous functional abnormalities after asymptomatic venous thrombosis following total hip or knee arthroplasty. Clin Orthop Relat Res. 1988;(232):271–278. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources