The HER2 inhibitor lapatinib potentiates doxorubicin-induced cardiotoxicity through iNOS signaling
- PMID: 29930721
- PMCID: PMC6010982
- DOI: 10.7150/thno.23207
The HER2 inhibitor lapatinib potentiates doxorubicin-induced cardiotoxicity through iNOS signaling
Abstract
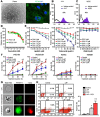
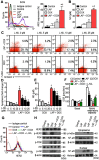
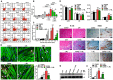
Rationale: Lapatinib (LAP) is a crucial alternative to trastuzumab upon the onset of drug resistance during treatment of metastatic human epidermal growth factor receptor 2-positive breast cancer. Like trastuzumab, LAP is commonly used alongside anthracyclines as a combination therapy, due to enhanced anti-cancer efficacy. However, this is notably associated with cardiotoxicity so it is imperative to understand the mechanisms driving this cardiotoxicity and develop cardioprotective strategies. To this end, here we utilize human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs), which exhibit several characteristics representative of in vivo cardiomyocytes that make them breakthrough models to study drug toxicity. Methods: We investigated LAP- and doxorubicin (DOX)-induced toxicity in hPSC-CMs and evaluated the involvement of inducible nitric oxide (NO) synthase (iNOS). The significance of iNOS-mediated cardiotoxicity was furthermore evaluated in animal studies. Results: LAP synergistically increased DOX toxicity in hPSC-CMs in a dose- and time-dependent manner. At concentrations that were otherwise non-apoptotic when administered separately, LAP significantly potentiated DOX-induced hPSC-CM apoptosis. This was accompanied by increased iNOS expression and pronounced production of NO. iNOS inhibition significantly reduced hPSC-CM sensitivity to LAP and DOX co-treatment (LAP-plus-DOX), leading to reduced apoptosis. Consistent with our observations in vitro, delivery of an iNOS inhibitor in mice treated with LAP-plus-DOX attenuated myocardial apoptosis and systolic dysfunction. Moreover, inhibition of iNOS did not compromise the anti-cancer potency of LAP-plus-DOX in a murine breast cancer xenograft model. Conclusions: Our findings suggest that iNOS inhibition is a promising cardioprotective strategy to accompany HER2-inhibitor/anthracycline combination therapies. Furthermore, these results support the promise of hPSC-CMs as a platform for investigating cardiotoxicity and developing cardioprotectants as a whole.
Keywords: HER2 inhibitor; cardiotoxicity; doxorubicin; iNOS; iPSC.
Conflict of interest statement
Competing Interests: Patrick Hsieh receives research grants from AstraZeneca, Gilead and Takeda, all of which did not participate in funding this work. The other authors report no conflicts.
Figures


Similar articles
-
Lapatinib induces mitochondrial dysfunction to enhance oxidative stress and ferroptosis in doxorubicin-induced cardiomyocytes via inhibition of PI3K/AKT signaling pathway.Bioengineered. 2022 Jan;13(1):48-60. doi: 10.1080/21655979.2021.2004980. Bioengineered. 2022. PMID: 34898356 Free PMC article.
-
Intravenous administration of cardiac progenitor cell-derived exosomes protects against doxorubicin/trastuzumab-induced cardiac toxicity.Cardiovasc Res. 2020 Feb 1;116(2):383-392. doi: 10.1093/cvr/cvz108. Cardiovasc Res. 2020. PMID: 31098627
-
Thousand and one amino acid protein kinase 1 suppression improves doxorubicin-induced cardiomyopathy by preventing cardiomyocyte death and dysfunction.Cardiovasc Res. 2025 May 6;121(4):601-613. doi: 10.1093/cvr/cvaf022. Cardiovasc Res. 2025. PMID: 39964965
-
An update of the molecular mechanisms underlying doxorubicin plus trastuzumab induced cardiotoxicity.Life Sci. 2021 Sep 1;280:119760. doi: 10.1016/j.lfs.2021.119760. Epub 2021 Jun 21. Life Sci. 2021. PMID: 34166713 Review.
-
Cardiotoxicity of novel HER2-targeted therapies.Curr Med Res Opin. 2013 Aug;29(8):1015-24. doi: 10.1185/03007995.2013.807232. Epub 2013 Jun 7. Curr Med Res Opin. 2013. PMID: 23692263 Review.
Cited by
-
Inhibition of miR-25 attenuates doxorubicin-induced apoptosis, reactive oxygen species production and DNA damage by targeting PTEN.Int J Med Sci. 2020 Jun 5;17(10):1415-1427. doi: 10.7150/ijms.41980. eCollection 2020. Int J Med Sci. 2020. PMID: 32624698 Free PMC article.
-
Screening for in-vivo regional contractile defaults to predict the delayed Doxorubicin Cardiotoxicity in Juvenile Rat.Theranostics. 2020 Jul 9;10(18):8130-8142. doi: 10.7150/thno.47407. eCollection 2020. Theranostics. 2020. PMID: 32724462 Free PMC article.
-
Cardiotoxicity of anthracycline-free targeted oncological therapies in HER2-positive breast cancer.Oncol Lett. 2021 Feb;21(2):100. doi: 10.3892/ol.2020.12361. Epub 2020 Dec 8. Oncol Lett. 2021. PMID: 33376533 Free PMC article. Review.
-
Human iPSC banking: barriers and opportunities.J Biomed Sci. 2019 Oct 28;26(1):87. doi: 10.1186/s12929-019-0578-x. J Biomed Sci. 2019. PMID: 31660969 Free PMC article. Review.
-
Toll-like receptor 5 deficiency diminishes doxorubicin-induced acute cardiotoxicity in mice.Theranostics. 2020 Sep 2;10(24):11013-11025. doi: 10.7150/thno.47516. eCollection 2020. Theranostics. 2020. PMID: 33042267 Free PMC article.
References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. - PubMed
-
- Stephens P, Hunter C, Bignell G, Edkins S, Davies H, Teague J. et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431:525–6. - PubMed
-
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE. et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. - PubMed
-
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T. et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

