Response to Single Low-dose 177Lu-DOTA-EB-TATE Treatment in Patients with Advanced Neuroendocrine Neoplasm: A Prospective Pilot Study
- PMID: 29930731
- PMCID: PMC6010978
- DOI: 10.7150/thno.25919
Response to Single Low-dose 177Lu-DOTA-EB-TATE Treatment in Patients with Advanced Neuroendocrine Neoplasm: A Prospective Pilot Study
Abstract
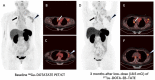
Objective:177Lu-DOTA-EB-TATE is a theranostic agent based on octreotate that uses an Evans blue structure to bind albumin to improve the pharmacokinetics and pharmacodynamics. This pilot study aims to evaluate the efficacy of a single low-dose treatment using 177Lu-DOTA-EB-TATE in patients with advanced neuroendocrine neoplasm (NEN). Methods: With IRB approval and informed consent, 4 NEN patients were enrolled to undergo 177Lu-DOTA-EB-TATE treatment with a single low dose of 0.66 ± 0.06 GBq (17.8 ± 1.7 mCi); 3 other NEN patients were enrolled as controls to undergo 177Lu-DOTA-TATE treatment with administered activity of 3.98 ± 0.17 GBq (107.6 ± 4.6 mCi). One primary tumor and 62 metastatic lesions in the 7 patients were evaluated by 68Ga-DOTA-TATE PET/CT immediately before and one or three months after the treatment. Maximum SUV (SUVmax) of the tumors ≥2.0 cm in diameter were measured and percentage of change (ΔSUV) after treatment were calculated. Results: All 4 patients subjected to 177Lu-DOTA-EB-TATE treatment tolerated the administered activity without significant adverse effects and showed symptomatic remission. Among the patients, 40 tumors were found with diameter ≥2.0 cm, with the baseline SUVmax varied from 1.5-82.9 (35.9 ± 21.0) and the ΔSUVs before and three months after the treatment from -75.1-26.3% (-38.9 ± 25.5%). Twenty-nine (72.5%) of the tumors showed >15% decrease of SUVmax (ΔSUV = -75.1%--17.1%). There was a significant negative correlation between the baseline SUVmax and the ΔSUV after treatment (r = -0.852, P < 0.001). Compared with the control 177Lu-DOTA-TATE therapy, the 177Lu-DOTA-EB-TATE treatment using approximately 1/6 the dose showed no significant difference in ΔSUV (-7.9 ± 5.4% vs. -5.8 ± 3.9%, P = 0.189) as demonstrated by the tumors with comparable baseline SUVmax from 10.0-35.0. Conclusion: A single low-dose 177Lu-DOTA-EB-TATE treatment appears to be safe and effective in the treatment of NENs with high 68Ga-DOTA-TATE uptake. This pilot study merits further investigation with increased dose and frequency of 177Lu-DOTA-EB-TATE administration with potential advantages over 177Lu-DOTA-TATE.
Keywords: 177Lu-DOTA-EB-TATE; 68Ga-DOTA-TATE; PET/CT; neuroendocrine neoplasm; treatment.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
Dose escalation of an Evans blue-modified radiolabeled somatostatin analog 177Lu-DOTA-EB-TATE in the treatment of metastatic neuroendocrine tumors.Eur J Nucl Med Mol Imaging. 2020 Apr;47(4):947-957. doi: 10.1007/s00259-019-04530-1. Epub 2019 Dec 12. Eur J Nucl Med Mol Imaging. 2020. PMID: 31832728 Free PMC article. Clinical Trial.
-
Safety, Pharmacokinetics, and Dosimetry of a Long-Acting Radiolabeled Somatostatin Analog 177Lu-DOTA-EB-TATE in Patients with Advanced Metastatic Neuroendocrine Tumors.J Nucl Med. 2018 Nov;59(11):1699-1705. doi: 10.2967/jnumed.118.209841. Epub 2018 Apr 13. J Nucl Med. 2018. PMID: 29653971 Free PMC article. Clinical Trial.
-
Evaluation of Safety, Biodistribution, and Dosimetry of a Long-Acting Radiolabeled Somatostatin Analog 177 Lu-DOTA-EB-TATE With and Without Amino Acid Infusion.Clin Nucl Med. 2023 Jun 1;48(6):e289-e293. doi: 10.1097/RLU.0000000000004642. Epub 2023 Apr 18. Clin Nucl Med. 2023. PMID: 37075254 Clinical Trial.
-
Overview of Development and Formulation of ¹⁷⁷Lu-DOTA-TATE for PRRT.Curr Radiopharm. 2016;9(1):8-18. doi: 10.2174/1874471008666150313111131. Curr Radiopharm. 2016. PMID: 25771369 Review.
-
Somatostatin receptor-targeted radionuclide therapy of tumors: preclinical and clinical findings.Semin Nucl Med. 2002 Apr;32(2):133-40. doi: 10.1053/snuc.2002.31027. Semin Nucl Med. 2002. PMID: 11965608 Review.
Cited by
-
Towards the Magic Radioactive Bullet: Improving Targeted Radionuclide Therapy by Reducing the Renal Retention of Radioligands.Pharmaceuticals (Basel). 2024 Feb 16;17(2):256. doi: 10.3390/ph17020256. Pharmaceuticals (Basel). 2024. PMID: 38399470 Free PMC article. Review.
-
Albumin Binder-Conjugated Fibroblast Activation Protein Inhibitor Radiopharmaceuticals for Cancer Therapy.J Nucl Med. 2022 Jun;63(6):952-958. doi: 10.2967/jnumed.121.262533. Epub 2021 Sep 30. J Nucl Med. 2022. PMID: 34593598 Free PMC article.
-
Peptide Receptor Radionuclide Therapy of Late-Stage Neuroendocrine Tumor Patients with Multiple Cycles of 177Lu-DOTA-EB-TATE.J Nucl Med. 2021 Mar;62(3):386-392. doi: 10.2967/jnumed.120.248658. Epub 2020 Aug 21. J Nucl Med. 2021. PMID: 32826319 Free PMC article.
-
The crucial role of age and site in incidence and prognosis of female neuroendocrine neoplasms in the United States: a population-based study from 2000 to 2018.Aging (Albany NY). 2024 Mar 1;16(5):4204-4223. doi: 10.18632/aging.205573. Epub 2024 Mar 1. Aging (Albany NY). 2024. PMID: 38431305 Free PMC article.
-
Molecular imaging and radionuclide therapy of pheochromocytoma and paraganglioma in the era of genomic characterization of disease subgroups.Endocr Relat Cancer. 2019 Nov;26(11):R627-R652. doi: 10.1530/ERC-19-0165. Endocr Relat Cancer. 2019. PMID: 31561209 Free PMC article. Review.
References
-
- Rindi G. The ENETS guidelines: the new TNM classification system. Tumori. 2010;96(5):806–809. - PubMed
-
- Anlauf M. Neuroendocrine neoplasms of the gastroenteropancreatic system: pathology and classification. Horm Metab Res. 2011;43(12):825–831. - PubMed
-
- Barakat MT, Meeran K, Bloom SR. Neuroendocrine tumors. Endocrine-related Cancer. 2004;11:1–18. - PubMed
-
- Modlin IM, Oberg K, Chung DC. et al. Gastroenteropancreatic neuroendocrine tumors. Lancet Oncol. 2008;9:61–72. - PubMed
-
- Plöckinger U, Rindi G, Arnold R. et al. European Neuroendocrine Tumor Society. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumors. A consensus statement on behalf of the European Neuroendocrine Tumor Society (ENETS) Neuroendocrinology. 2004;80:394–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

