Injectable OPF/graphene oxide hydrogels provide mechanical support and enhance cell electrical signaling after implantation into myocardial infarct
- PMID: 29930732
- PMCID: PMC6010993
- DOI: 10.7150/thno.25504
Injectable OPF/graphene oxide hydrogels provide mechanical support and enhance cell electrical signaling after implantation into myocardial infarct
Abstract
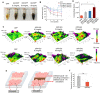
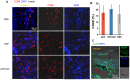
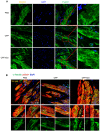
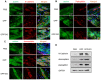
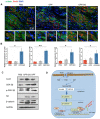
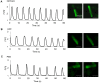
After myocardial infarction (MI), the scar tissue contributes to ventricular dysfunction by electrically uncoupling viable cardiomyocytes in the infarct region. Injection of a conductive hydrogel could not only provide mechanical support to the infarcted region, but also synchronize contraction and restore ventricular function by electrically connecting isolated cardiomyocytes to intact tissue. Methods: We created a conductive hydrogel by introducing graphene oxide (GO) nanoparticles into oligo(poly(ethylene glycol) fumarate) (OPF) hydrogels. The hydrogels were characterized by AFM and electrochemistry workstation. A rat model of myocardial infarction was used to investigate the ability of OPF/GO to improve cardiac electrical propagation in the injured heart in vivo. Echocardiography (ECHO) was used to evaluate heart function 4 weeks after MI. Ca2+ imaging was used to visualize beating cardiomyocytes (CMs). Immunofluorescence staining was used to visualize the expression of cardiac-specific markers. Results: OPF/GO hydrogels had semiconductive properties that were lacking in pure OPF. In addition, the incorporation of GO into OPF hydrogels could improve cell attachment in vitro. Injection of OPF/GO 4 weeks after myocardial infarction in rats enhanced the Ca2+ signal conduction of cardiomyocytes in the infarcted region in comparison with PBS or OPF alone. Moreover, the injection of OPF/GO hydrogel into the infarct region enhanced the generation of cytoskeletal structure and intercalated disc assembly. Echocardiography analysis showed improvement in load-dependent ejection fraction/fractional shortening of heart function 4 weeks after injection. Conclusions: We prepared a conductive hydrogel (OPF/GO) that provide mechanical support and biological conduction in vitro and in vivo. We found that injected OPF/GO hydrogels can provide mechanical support and electric connection between healthy myocardium and the cardiomyocytes in the scar via activating the canonical Wnt signal pathway, thus upregulating the generation of Cx43 and gap junction associated proteins. Injection of OPF/GO hydrogel maintained better heart function after myocardial infarction than the injection of a nonconductive polymer.
Keywords: conduction; injectable biomaterials; myocardial infarction; remodeling.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Davis S, Norrving B. Organizational update: world stroke organization. Stroke. 2015;46:e9–e10. - PubMed
-
- Hao T, Li J, Yao F. et al. Injectable fullerenol/alginate hydrogel for suppression of oxidative stress damage in brown adipose-derived stem cells and cardiac repair. ACS Nano. 2017;11:5474–5488. - PubMed
-
- Henning RJ, Khan A, Jimenez E. Chitosan hydrogels significantly limit left ventricular infarction and remodeling and preserve myocardial contractility. J Surg Res. 2016;201:490–497. - PubMed
-
- Palmer CR. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. New Engl J Med. 2013;369:1395–1405. - PubMed
-
- Russo V, Young S, Hamilton A. et al. Mesenchymal stem cell delivery strategies to promote cardiac regeneration following ischemic injury. Biomaterials. 2014;35:3956–3974. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

