Extracellular Vesicles as Markers and Mediators in Sepsis
- PMID: 29930734
- PMCID: PMC6010985
- DOI: 10.7150/thno.23453
Extracellular Vesicles as Markers and Mediators in Sepsis
Abstract
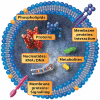
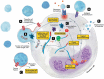
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. It remains a highly lethal condition in which current tools for early diagnosis and therapeutic decision-making are far from ideal. Extracellular vesicles (EVs), 30 nm to several micrometers in size, are released from cells upon activation and apoptosis and express membrane epitopes specific for their parental cells. Since their discovery two decades ago, their role as biomarkers and mediators in various diseases has been intensively studied. However, their potential importance in the sepsis syndrome has gained attention only recently. Sepsis and EVs are both complex fields in which standardization has long been overdue. In this review, several topics are discussed. First, we review current studies on EVs in septic patients with emphasis on their variable quality and clinical utility. Second, we discuss the diagnostic and therapeutic potential of EVs as well as their role as facilitators of cell communication via micro RNA and the relevance of micro-organism-derived EVs. Third, we give an overview over the potential beneficial but also detrimental roles of EVs in sepsis. Finally, we focus on the role of EVs in selected intensive care scenarios such as coagulopathy, mechanical ventilation and blood transfusion. Overall, the prospect for EV use in septic patients is bright, ranging from rapid and precise (point-of-care) diagnostics, prevention of harmful iatrogenic interventions, to using EVs as guides of individualized therapy. Before the above is achieved, however, the EV research field requires reliable standardization of the current methods and development of new analytical procedures that can close the existing technological gaps.
Keywords: exosomes; inflammation; microparticles; point-of-care; sepsis.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Extracellular vesicles participate in the pathogenesis of sepsis.Front Cell Infect Microbiol. 2022 Dec 12;12:1018692. doi: 10.3389/fcimb.2022.1018692. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36579343 Free PMC article. Review.
-
Extracellular Vesicles in Cardiovascular Theranostics.Theranostics. 2017 Sep 26;7(17):4168-4182. doi: 10.7150/thno.21274. eCollection 2017. Theranostics. 2017. PMID: 29158817 Free PMC article. Review.
-
Extracellular Vesicles: A Brief Overview and Its Role in Precision Medicine.Methods Mol Biol. 2017;1660:1-14. doi: 10.1007/978-1-4939-7253-1_1. Methods Mol Biol. 2017. PMID: 28828643 Review.
-
Extracellular vesicles and their nucleic acids for biomarker discovery.Pharmacol Ther. 2018 Dec;192:170-187. doi: 10.1016/j.pharmthera.2018.08.002. Epub 2018 Aug 3. Pharmacol Ther. 2018. PMID: 30081050 Review.
-
Extracellular Vesicle as a Source of Alzheimer's Biomarkers: Opportunities and Challenges.Int J Mol Sci. 2019 Apr 8;20(7):1728. doi: 10.3390/ijms20071728. Int J Mol Sci. 2019. PMID: 30965555 Free PMC article. Review.
Cited by
-
Y-RNA subtype ratios in plasma extracellular vesicles are cell type- specific and are candidate biomarkers for inflammatory diseases.J Extracell Vesicles. 2020 May 26;9(1):1764213. doi: 10.1080/20013078.2020.1764213. J Extracell Vesicles. 2020. PMID: 32944168 Free PMC article.
-
Circular RNA mmu_circ_0001295 from hypoxia pretreated adipose-derived mesenchymal stem cells (ADSCs) exosomes improves outcomes and inhibits sepsis-induced renal injury in a mouse model of sepsis.Bioengineered. 2022 Mar;13(3):6323-6331. doi: 10.1080/21655979.2022.2044720. Bioengineered. 2022. PMID: 35212606 Free PMC article.
-
Prognostic implication of downregulated exosomal miRNAs in patients with sepsis: a cross-sectional study with bioinformatics analysis.J Intensive Care. 2023 Aug 3;11(1):35. doi: 10.1186/s40560-023-00683-2. J Intensive Care. 2023. PMID: 37537685 Free PMC article.
-
Low expression of CD39 on monocytes predicts poor survival in sepsis patients.J Intensive Care. 2025 Mar 10;13(1):12. doi: 10.1186/s40560-025-00784-0. J Intensive Care. 2025. PMID: 40065471 Free PMC article.
-
Unveiling the Role of Exosomes in the Pathophysiology of Sepsis: Insights into Organ Dysfunction and Potential Biomarkers.Int J Mol Sci. 2024 Apr 30;25(9):4898. doi: 10.3390/ijms25094898. Int J Mol Sci. 2024. PMID: 38732114 Free PMC article. Review.
References
-
- Fleischmann C, Scherag A. et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Crit Care Med. 2016;193:259–272. - PubMed
-
- Rhodes A, Evans LE, Alhazzani W. et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. - PubMed
-
- Walley KR. Biomarkers in sepsis. Curr Infect Dis Rep. 2013;15:413–420. - PubMed
-
- Fisher CJ Jr, Slotman GJ, Opal SM. et al. Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: a randomized, open-label, placebo-controlled multicenter trial. Crit Care Med. 1994;22:12–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

