Pediatric Inflammatory Bowel Disease Clinical Innovations Meeting of the Crohn's & Colitis Foundation: Charting the Future of Pediatric IBD
- PMID: 29931102
- PMCID: PMC8133504
- DOI: 10.1093/ibd/izy205
Pediatric Inflammatory Bowel Disease Clinical Innovations Meeting of the Crohn's & Colitis Foundation: Charting the Future of Pediatric IBD
Abstract
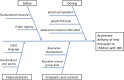
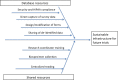
The Crohn's & Colitis Foundation has facilitated transformational research in pediatric inflammatory bowel disease (IBD), through the RISK and PROTECT studies, that has laid the groundwork for a comprehensive understanding of molecular mechanisms of disease and predictors of therapeutic response in children. Despite these advances, children have lacked timely and informed access to the latest therapeutic advancements in IBD. The Crohn's & Colitis Foundation convened a Pediatric Resource Organization for Kids with Inflammatory Intestinal Diseases (PRO-KIIDS) Clinical Innovations Meeting at the inaugural Crohn's and Colitis Congress in January 2018 to devise how to advance the care of children with IBD. The working group selected 2 priorities: (1) accelerating therapies to children with IBD and (2) stimulating investigator-initiated research while fostering sustainable collaboration; and proposed 2 actions: (a) the convening of a task force to specifically address how to accelerate pharmacotherapies to children with IBD and (b) the funding of a multicenter clinical and translational research study that incorporates the building of critical research infrastructure.10.1093/ibd/izy205_video1izy205.video15799266615001.
Figures
Similar articles
-
Management of inflammatory bowel disease with infliximab and other anti-tumor necrosis factor alpha therapies.BioDrugs. 2010 Dec 14;24 Suppl 1:3-14. doi: 10.2165/11586290-000000000-00000. BioDrugs. 2010. PMID: 21175228 Review.
-
Antitumor necrosis factor treatment for pediatric inflammatory bowel disease.Inflamm Bowel Dis. 2012 May;18(5):985-1002. doi: 10.1002/ibd.21871. Epub 2011 Sep 20. Inflamm Bowel Dis. 2012. PMID: 21936033 Review.
-
European Crohn's and Colitis Organisation Topical Review on IBD in the Elderly.J Crohns Colitis. 2017 Mar 1;11(3):263-273. doi: 10.1093/ecco-jcc/jjw188. Epub 2016 Oct 20. J Crohns Colitis. 2017. PMID: 27797918
-
The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: The use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease.Dig Liver Dis. 2011 Jan;43(1):1-20. doi: 10.1016/j.dld.2010.07.010. Epub 2010 Sep 16. Dig Liver Dis. 2011. PMID: 20843756
-
Concomitant therapy with methotrexate and anti-TNF-α in pediatric patients with refractory crohn's colitis: a case series.Inflamm Bowel Dis. 2012 Aug;18(8):1488-92. doi: 10.1002/ibd.21885. Epub 2011 Aug 31. Inflamm Bowel Dis. 2012. PMID: 21882301
Cited by
-
Paediatric Inflammatory Bowel Disease: A Multi-Stakeholder Perspective to Improve Development of Drugs for Children and Adolescents.J Crohns Colitis. 2023 Mar 18;17(2):249-258. doi: 10.1093/ecco-jcc/jjac135. J Crohns Colitis. 2023. PMID: 36130314 Free PMC article.
-
Developmental Pharmacodynamics and Modeling in Pediatric Drug Development.J Clin Pharmacol. 2019 Sep;59 Suppl 1(Suppl 1):S87-S94. doi: 10.1002/jcph.1482. J Clin Pharmacol. 2019. PMID: 31502687 Free PMC article. Review.
-
Development of the CDISC Pediatrics User Guide: a CDISC and conect4children collaboration.Front Med (Lausanne). 2024 Jun 17;11:1370916. doi: 10.3389/fmed.2024.1370916. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38966540 Free PMC article.
-
Natural History of Very Early Onset Inflammatory Bowel Disease in North America: A Retrospective Cohort Study.Inflamm Bowel Dis. 2021 Feb 16;27(3):295-302. doi: 10.1093/ibd/izaa080. Inflamm Bowel Dis. 2021. PMID: 32386060 Free PMC article.
-
Identification of suitable reference microRNA for qPCR analysis in pediatric inflammatory bowel disease.Physiol Genomics. 2019 May 1;51(5):169-175. doi: 10.1152/physiolgenomics.00126.2018. Epub 2019 Apr 12. Physiol Genomics. 2019. PMID: 30978148 Free PMC article.
References
-
- Baldassano RN, Piccoli DA. Inflammatory bowel disease in pediatric and adolescent patients. Gastroenterol Clin North Am. 1999;28:445–458. - PubMed
-
- North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition, Colitis Foundation of America , Bousvaros A, et al. . Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44:653–674. - PubMed
-
- Griffiths AM, Otley AR, Hyams J, et al. . A review of activity indices and end points for clinical trials in children with Crohn’s disease. Inflamm Bowel Dis. 2005;11:185–196. - PubMed
-
- Turner D, Otley AR, Mack D, et al. . Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423–432. - PubMed
-
- Bousvaros A, Sylvester F, Kugathasan S, et al. ; Challenges in Pediatric IBD Study Groups . Challenges in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:885–913. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical