Mammalian Cells Engineered To Produce New Steroids
- PMID: 29931794
- PMCID: PMC6156985
- DOI: 10.1002/cbic.201800214
Mammalian Cells Engineered To Produce New Steroids
Abstract
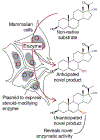
Steroids can be difficult to modify through traditional organic synthesis methods, but many enzymes regio- and stereoselectively process a wide variety of steroid substrates. We tested whether steroid-modifying enzymes could make novel steroids from non-native substrates. Numerous genes encoding steroid-modifying enzymes, including some bacterial enzymes, were expressed in mammalian cells by transient transfection and found to be active. We made three unusual steroids by stable expression, in HEK293 cells, of the 7α-hydroxylase CYP7B1, which was selected because of its high native product yield. These cells made 7α,17α-dihydroxypregnenolone and 7β,17α-dihydroxypregnenolone from 17α-hydroxypregnenolone and produced 11α,16α-dihydroxyprogesterone from 16α-hydroxyprogesterone. The last two products were the result of CYP7B1-catalyzed hydroxylation at previously unobserved sites. A Rosetta docking model of CYP7B1 suggested that these substrates' D-ring hydroxy groups might prevent them from binding in the same way as the native substrates, bringing different carbon atoms close to the active ferryl oxygen atom. This new approach could potentially use other enzymes and substrates to produce many novel steroids for drug candidate testing.
Keywords: biosynthesis; cytochromes; hydroxylation; steroids; synthetic biology.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


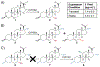
References
-
- Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D, Clin. Ther 2011, 33, 1413–1432. - PubMed
-
- Haendler B, Cleve A, Mol. Cell. Endocrinol 2012, 352, 79–91. - PubMed
-
- Mager DE, Moledina N, Jusko WJ, J. Pharm. Sci 2003, 92, 1521–1525. - PubMed
-
- Keski-Rahkonen P, Huhtinen K, Poutanen M, Auriola S, J. Steroid Biochem. Mol. Biol 2011, 127, 396–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

