Effects of living at moderate altitude on pulmonary vascular function and exercise capacity in mice with sickle cell anaemia
- PMID: 29931797
- PMCID: PMC6376072
- DOI: 10.1113/JP275810
Effects of living at moderate altitude on pulmonary vascular function and exercise capacity in mice with sickle cell anaemia
Abstract
Key points: Sickle cell disease (SCD) results in cardiopulmonary dysfunction, which may be exacerbated by prolonged exposure to environmental hypoxia. It is currently unknown whether exposure to mild and moderate altitude exacerbates SCD associated cardiopulmonary and systemic complications. Three months of exposure to mild (1609 m) and moderate (2438 m) altitude increased rates of haemolysis and right ventricular systolic pressures in mice with SCD compared to healthy wild-type cohorts and SCD mice at sea level. The haemodynamic changes in SCD mice that had lived at mild and moderate altitude were accompanied by changes in the balance between pulmonary vascular endothelial nitric oxide synthase and endothelin receptor expression and impaired exercise tolerance. These data demonstrate that chronic altitude exposure exacerbates the complications associated with SCD and provides pertinent information for the clinical counselling of SCD patients.
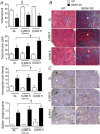
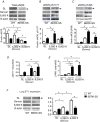
Abstract: Exposure to high altitude worsens symptoms and crises in patients with sickle cell disease (SCD). However, it remains unclear whether prolonged exposure to low barometric pressures exacerbates SCD aetiologies or impairs quality of life. We tested the hypothesis that, relative to wild-type (WT) mice, Berkley sickle cell mice (BERK-SS) residing at sea level, mild (1609 m) and moderate (2438 m) altitude would have a higher rate of haemolysis, impaired cardiac function and reduced exercise tolerance, and that the level of altitude would worsen these decrements. Following 3 months of altitude exposure, right ventricular systolic pressure was measured (solid-state transducer). In addition, the adaptive balance between pulmonary vascular endothelial nitric oxide synthase and endothelin was assessed in lung tissue to determine differences in pulmonary vascular adaptation and the speed/duration relationship (critical speed) was used to evaluate treadmill exercise tolerance. At all altitudes, BERK-SS mice had a significantly lower percentage haemocrit and higher total bilirubin and free haemoglobin concentration (P < 0.05 for all). right ventricular systolic pressures in BERK-SS were higher than WT at moderate altitude and also compared to BERK-SS at sea level (P < 0.05, for both). Critical speed was significantly lower in BERK-SS at mild and moderate altitude (P < 0.05). BERK-SS demonstrated exacerbated SCD complications and reduced exercise capacity associated with an increase in altitude. These results suggest that exposure to mild and moderate altitude enhances the progression of SCD in BERK-SS mice compared to healthy WT cohorts and BERK-SS mice at sea level and provides crucial information for the clinical counselling of SCD patients.
Keywords: BERK mouse; critical speed; hemolysis; hypoxia.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures



Comment in
-
Sickle cell vasculopathy: vascular phenotype on fire!J Physiol. 2019 Feb;597(4):993-994. doi: 10.1113/JP276705. Epub 2018 Aug 3. J Physiol. 2019. PMID: 30007009 Free PMC article. No abstract available.
References
-
- Bakeer N, James J, Roy S, Wansapura J, Shanmukhappa SK, Lorenz JN, Osinska H, Backer K, Huby AC, Shrestha A, Niss O, Fleck R, Quinn CT, Taylor MD, Purevjav E, Aronow BJ, Towbin JA & Malik P (2016). Sickle cell anemia mice develop a unique cardiomyopathy with restrictive physiology. Proc Natl Acad Sci U S A 113, E5182‐5191. - PMC - PubMed
-
- Barst RJ, Mubarak KK, Machado RF, Ataga KI, Benza RL, Castro O, Naeije R, Sood N, Swerdlow PS & Hildesheim M (2010). Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies. Br J Haematol 149, 426–435. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

