The Quality of Informed Consent Forms-a Systematic Review and Critical Analysis
- PMID: 29932049
- PMCID: PMC6039714
- DOI: 10.3238/arztebl.2018.0377
The Quality of Informed Consent Forms-a Systematic Review and Critical Analysis
Abstract
Background: The patient's consent to a medical procedure must be preceded by a pre-procedure discussion with the physician that is documented on a standardized form. Evidence suggests that these forms lack information that would be relevant for an informed decision.
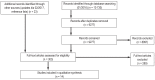
Methods: We carried out a systematic literature search up to February 2017 for evidence on the quality and efficacy of informed consent forms. The definition of criteria for the evaluation of meta-information, content, and presentation were derived from current guidelines for evidence-based health information. As an example, we analyzed consent forms currently in use in Germany for 10 medical interventions with regard to decisionally relevant content and intelligibility of format.
Results: Our literature search yielded 14 content analyses, which revealed that even some of the more important evaluative criteria were not always met, including information on benefits (9/14), risks (14/14), alternatives (11/14), the option of doing nothing (6/14), and numerical frequencies (2/14). All analyses indicated deficiencies in the content of the consent forms. We then analyzed 37 consent forms obtained from publishing companies (across Germany) and physician's practices in Hamburg. These forms were found to contain information on: the intervention (37/37), benefits (30/37), risks (37/37), alternatives (26/37), the option of doing nothing (4/37), numerical frequencies (10/37), the names of the authors (17/37), sources of information (0/37), and date of issue (21/37).
Conclusion: Both the evidence from foreign countries and our own analysis of the consent forms now in use in Germany revealed deficiencies, particularly in the communication of risks. New standards are needed to promote well-informed decision-making. Structural changes in the process of patient information and decision-making should be discussed.
Figures
Comment in
-
Validity of Evaluation Criteria Is Not Clear.Dtsch Arztebl Int. 2018 Sep 21;115(38):636. doi: 10.3238/arztebl.2018.0636a. Dtsch Arztebl Int. 2018. PMID: 30373710 Free PMC article. No abstract available.
-
Time Expenditure as a Quality Criterion.Dtsch Arztebl Int. 2018 Sep 21;115(38):636-637. doi: 10.3238/arztebl.2018.0636b. Dtsch Arztebl Int. 2018. PMID: 30373711 Free PMC article. No abstract available.
References
-
- Bundesärztekammer. (Muster-)Berufsordnung für die in Deutschland tätigen Ärztinnen und Ärzte - MBO-Ä 1997 - in der Fassung des Beschlusses des 118. Deutschen Ärztetages 2015 in Frankfurt am Main. Dtsch Arztebl. 2015;112 A-1348.
-
- Oberlandesgericht (OLG) Köln. Urteil vom 26. Oktober 2011 (Az. 5 U 46/11) www.openjur.de/u/451468.html (last accessed on 26 September 2017)
-
- Oberlandesgericht (OLG) Hamm. Urteil vom 18. Juni 2013 (AZ. 26 U 85/12) www.openjur.de/u/645241.html (last accessed on 26 September 2017)
-
- Loughran D. Surgical consent: the world‘s largest Chinese whisper? A review of current surgical consent practices. Med Ethics. 2015;41:206–210. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous