Regulation of Cellular Metabolism by High-Risk Human Papillomaviruses
- PMID: 29932118
- PMCID: PMC6073392
- DOI: 10.3390/ijms19071839
Regulation of Cellular Metabolism by High-Risk Human Papillomaviruses
Abstract
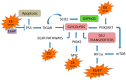
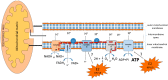
The alteration of glucose metabolism is one of the first biochemical characteristics associated with cancer cells since most of these cells increase glucose consumption and glycolytic rates even in the presence of oxygen, which has been called “aerobic glycolysis” or the Warburg effect. Human papillomavirus (HPV) is associated with approximately 5% of all human cancers worldwide, principally to cervical cancer. E6 and E7 are the main viral oncoproteins which are required to preserve the malignant phenotype. These viral proteins regulate the cell cycle through their interaction with tumor suppressor proteins p53 and pRB, respectively. Together with the viral proteins E5 and E2, E6 and E7 can favor the Warburg effect and contribute to radio- and chemoresistance through the increase in the activity of glycolytic enzymes, as well as the inhibition of the Krebs cycle and the respiratory chain. These processes lead to a fast production of ATP obtained by Warburg, which could help satisfy the high energy demands of cancer cells during proliferation. In this way HPV proteins could promote cancer hallmarks. However, it is also possible that during an early HPV infection, the Warburg effect could help in the achievement of an efficient viral replication.
Keywords: Warburg effect; human papillomavirus; metabolism; oxidative phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
The regulation of cell proliferation by the papillomavirus early proteins.Cell Mol Life Sci. 2009 May;66(10):1700-17. doi: 10.1007/s00018-009-8631-7. Cell Mol Life Sci. 2009. PMID: 19183849 Free PMC article. Review.
-
Tanshinone IIA inhibits viral oncogene expression leading to apoptosis and inhibition of cervical cancer.Cancer Lett. 2015 Jan 28;356(2 Pt B):536-46. doi: 10.1016/j.canlet.2014.09.037. Epub 2014 Oct 7. Cancer Lett. 2015. PMID: 25304375
-
Signaling pathways in HPV-associated cancers and therapeutic implications.Rev Med Virol. 2015 Mar;25 Suppl 1:24-53. doi: 10.1002/rmv.1823. Rev Med Virol. 2015. PMID: 25752815 Review.
-
Novel Functions of the Human Papillomavirus E6 Oncoproteins.Annu Rev Virol. 2015 Nov;2(1):403-23. doi: 10.1146/annurev-virology-100114-055021. Epub 2015 Sep 2. Annu Rev Virol. 2015. PMID: 26958922 Review.
-
Induction of dormancy in hypoxic human papillomavirus-positive cancer cells.Proc Natl Acad Sci U S A. 2017 Feb 7;114(6):E990-E998. doi: 10.1073/pnas.1615758114. Epub 2017 Jan 23. Proc Natl Acad Sci U S A. 2017. PMID: 28115701 Free PMC article.
Cited by
-
HIV-1 Reverse Transcriptase Expression in HPV16-Infected Epidermoid Carcinoma Cells Alters E6 Expression and Cellular Metabolism, and Induces a Hybrid Epithelial/Mesenchymal Cell Phenotype.Viruses. 2024 Jan 26;16(2):193. doi: 10.3390/v16020193. Viruses. 2024. PMID: 38399969 Free PMC article.
-
Targeting Mitochondrial Therapy in the Regulation of HPV Infection and HPV-Related Cancers.Pathogens. 2023 Mar 2;12(3):402. doi: 10.3390/pathogens12030402. Pathogens. 2023. PMID: 36986324 Free PMC article. Review.
-
Human Papilloma Virus: An Unraveled Enigma of Universal Burden of Malignancies.Pathogens. 2023 Apr 6;12(4):564. doi: 10.3390/pathogens12040564. Pathogens. 2023. PMID: 37111450 Free PMC article. Review.
-
Bioinformatics Analysis Reveals E6 and E7 of HPV 16 Regulate Metabolic Reprogramming in Cervical Cancer, Head and Neck Cancer, and Colorectal Cancer through the PHD2-VHL-CUL2-ELOC-HIF-1α Axis.Curr Issues Mol Biol. 2024 Jun 19;46(6):6199-6222. doi: 10.3390/cimb46060370. Curr Issues Mol Biol. 2024. PMID: 38921041 Free PMC article.
-
Viral Reprogramming of Nucleotide Synthesis and Its Impact on Viral Infection.J Med Virol. 2025 Aug;97(8):e70563. doi: 10.1002/jmv.70563. J Med Virol. 2025. PMID: 40832900 Free PMC article. Review.
References
-
- Berg J.M., Jeremy M., Tymoczko J.L., Stryer L., Stryer L. Biochemistry. W.H. Freeman; New York, NY, USA: 2002.
-
- Semenza G.L., Artemov D., Bedi A., Bhujwalla Z., Chiles K., Feldser D., Laughner E., Ravi R., Simons J., Taghavi P., et al. “The metabolism of tumours”: 70 Years later. Novartis Found. Symp. 2001;240:251–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

