Hypoxia negatively affects senescence in osteoclasts and delays osteoclastogenesis
- PMID: 29932209
- PMCID: PMC6220985
- DOI: 10.1002/jcp.26511
Hypoxia negatively affects senescence in osteoclasts and delays osteoclastogenesis
Abstract
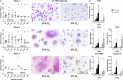
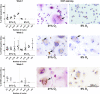
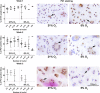
Cellular senescence, that is, the withdrawal from the cell cycle, combined with the acquirement of the senescence associated secretory phenotype has important roles during health and disease and is essential for tissue remodeling during embryonic development. Osteoclasts are multinucleated cells, responsible for bone resorption, and cell cycle arrest during osteoclastogenesis is well recognized. Therefore, the aim of this study was to investigate whether these cells should be considered senescent and to assess the influence of hypoxia on their potential senescence status. Osteoclastogenesis and bone resorption capacity of osteoclasts, cultured from CD14+ monocytes, were evaluated in two oxygen concentrations, normoxia (21% O2 ) and hypoxia (5% O2 ). Osteoclasts were profiled by using specific staining for proliferation and senescence markers, qPCR of a number of osteoclast and senescence-related genes and a bone resorption assay. Results show that during in vitro osteoclastogenesis, osteoclasts heterogeneously obtain a senescent phenotype. Furthermore, osteoclastogenesis was delayed at hypoxic compared to normoxic conditions, without negatively affecting the bone resorption capacity. It is concluded that osteoclasts can be considered senescent, although senescence is not uniformly present in the osteoclast population. Hypoxia negatively affects the expression of some senescence markers. Based on the direct relationship between senescence and osteoclastogenesis, it is tempting to hypothesize that contents of the so-called senescence associated secretory phenotype (SASP) not only play a functional role in matrix resorption, but also may regulate osteoclastogenesis.
Keywords: cellular senescence; hypoxia; osteoclastogenesis; osteoclasts.
© 2018 The Authors. Journal of Cellular Physiology Published by Wiley Periodicals Inc.
Figures





Similar articles
-
Constant hypoxia inhibits osteoclast differentiation and bone resorption by regulating phosphorylation of JNK and IκBα.Inflamm Res. 2019 Feb;68(2):157-166. doi: 10.1007/s00011-018-1209-9. Epub 2019 Jan 2. Inflamm Res. 2019. PMID: 30604211
-
Evidence for altered osteoclastogenesis in splenocyte cultures from Cyp27b1 knockout mice.J Steroid Biochem Mol Biol. 2016 Nov;164:353-360. doi: 10.1016/j.jsbmb.2015.11.015. Epub 2015 Nov 27. J Steroid Biochem Mol Biol. 2016. PMID: 26639637
-
Irradiation-induced senescence of bone marrow mesenchymal stem cells aggravates osteogenic differentiation dysfunction via paracrine signaling.Am J Physiol Cell Physiol. 2020 May 1;318(5):C1005-C1017. doi: 10.1152/ajpcell.00520.2019. Epub 2020 Apr 1. Am J Physiol Cell Physiol. 2020. PMID: 32233952
-
Overview of RAW264.7 for osteoclastogensis study: Phenotype and stimuli.J Cell Mol Med. 2019 May;23(5):3077-3087. doi: 10.1111/jcmm.14277. Epub 2019 Mar 20. J Cell Mol Med. 2019. PMID: 30892789 Free PMC article. Review.
-
Osteoclast formation at the bone marrow/bone surface interface: Importance of structural elements, matrix, and intercellular communication.Semin Cell Dev Biol. 2021 Apr;112:8-15. doi: 10.1016/j.semcdb.2020.05.016. Epub 2020 Jun 17. Semin Cell Dev Biol. 2021. PMID: 32563679 Review.
Cited by
-
Ageing-related bone and immunity changes: insights into the complex interplay between the skeleton and the immune system.Bone Res. 2024 Aug 5;12(1):42. doi: 10.1038/s41413-024-00346-4. Bone Res. 2024. PMID: 39103328 Free PMC article. Review.
-
Mechanisms and significance of therapy-induced and spontaneous senescence of cancer cells.Cell Mol Life Sci. 2020 Jan;77(2):213-229. doi: 10.1007/s00018-019-03261-8. Epub 2019 Aug 14. Cell Mol Life Sci. 2020. PMID: 31414165 Free PMC article. Review.
-
Hypoxia Promotes Osteoclast Differentiation by Weakening USP18-Mediated Suppression on the NF-κB Signaling Pathway.Int J Mol Sci. 2024 Dec 24;26(1):10. doi: 10.3390/ijms26010010. Int J Mol Sci. 2024. PMID: 39795869 Free PMC article.
-
Exercise to Mend Aged-tissue Crosstalk in Bone Targeting Osteoporosis & Osteoarthritis.Semin Cell Dev Biol. 2022 Mar;123:22-35. doi: 10.1016/j.semcdb.2021.08.011. Epub 2021 Sep 4. Semin Cell Dev Biol. 2022. PMID: 34489173 Free PMC article. Review.
-
Senescence of endplate osteoclasts induces sensory innervation and spinal pain.Elife. 2024 Jun 19;12:RP92889. doi: 10.7554/eLife.92889. Elife. 2024. PMID: 38896465 Free PMC article.
References
-
- Andersen, C. L. , Jensen, J. L. , & Ørntoft, T. F. (2004). Normalization of real‐time quantitative reverse transcription‐PCR data: A model‐based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Research, 64, 5245–5250. - PubMed
-
- Arnett, T. R. , Gibbons, D. C. , Utting, J. C. , Orriss, I. R. , Hoebertz, A. , Rosendaal, M. , & Meghji, S. (2003). Hypoxia is a major stimulator of osteoclast formation and bone resorption. Journal of Cellular Physiology, 196, 2–8. - PubMed
-
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57, 289–300.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

