Shaping the flavivirus replication complex: It is curvaceous!
- PMID: 29933527
- PMCID: PMC7162344
- DOI: 10.1111/cmi.12884
Shaping the flavivirus replication complex: It is curvaceous!
Abstract
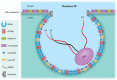
Flavivirus replication is intimately involved with remodelled membrane organelles that are compartmentalised for different functions during their life cycle. Recent advances in lipid analyses and gene depletion have identified a number of host components that enable efficient virus replication in infected cells. Here, we describe the current understanding on the role and contribution of host lipids and membrane bending proteins to flavivirus replication, with a particular focus on the components that bend and shape the membrane bilayer to induce the flavivirus-induced organelles characteristic of infection.
Keywords: flavivirus replication; lipids; membrane curvature; replication complex.
© 2018 John Wiley & Sons Ltd.
Figures
References
-
- Aktepe, T. E. , Liebscher, S. , Prier, J. E. , Simmons, C. P. , & Mackenzie, J. M. (2017). The host protein reticulon 3.1 A is utilized by flaviviruses to facilitate membrane remodelling. Cell Reports, 21(6), 1639–1654. - PubMed
-
- Aktepe, T. E. , Pham, H. , & Mackenzie, J. M. (2015). Differential utilisation of ceramide during replication of the flaviviruses West Nile and dengue virus. Virology, 484, 241–250. - PubMed
-
- Berger, K. L. , Cooper, J. D. , Heaton, N. S. , Yoon, R. , Oakland, T. E. , Jordan, T. X. , … Randall, G. (2009). Roles for endocytic trafficking and phosphatidylinositol 4‐kinase III alpha in hepatitis C virus replication. Proceedings of the National Academy of Sciences, 106(18), 7577–7582. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

