Synthesis of Porous CoFe₂O₄ and Its Application as a Peroxidase Mimetic for Colorimetric Detection of H₂O₂ and Organic Pollutant Degradation
- PMID: 29933545
- PMCID: PMC6071025
- DOI: 10.3390/nano8070451
Synthesis of Porous CoFe₂O₄ and Its Application as a Peroxidase Mimetic for Colorimetric Detection of H₂O₂ and Organic Pollutant Degradation
Abstract
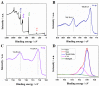
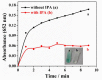
Porous CoFe₂O₄ was prepared via a simple and controllable method to develop a low-cost, high-efficiency, and good-stability nanozyme. The morphology and microstructure of the obtained CoFe₂O₄ was investigated by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), high-resolution TEM (HRTEM), specific surface area and pore analysis, and Raman spectroscopy. The results show that the annealing temperature has an important effect on the crystallinity, grain size, and specific surface area of CoFe₂O₄. CoFe₂O₄ obtained at 300 °C (CF300) exhibits the largest surface area (up to 204.1 m² g−1) and the smallest grain size. The peroxidase-like activity of CoFe₂O₄ was further verified based on the oxidation of peroxidase substrate 3,3’,5,5’-tetramethylbenzidine (TMB) in the presence of H₂O₂. The best peroxidase-like activity for CF300 should be ascribed to its largest surface area and smallest grain size. On this basis, an effective method of colorimetric detection H₂O₂ was established. In addition, the porous CoFe₂O₄ was also used for the catalytic oxidation of methylene blue (MB), indicating potential applications in pollutant removal and water treatment.
Keywords: CoFe2O4; artificial enzyme mimetics; hydrogen peroxide; peroxidase-like activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Wang N., Sun J., Chen L., Fan H., Ai S. A Cu2(OH)3Cl-CeO2 nanocomposite with peroxidase-like activity, and its application to the determination of hydrogen peroxide, glucose and cholesterol. Microchim. Acta. 2015;182:1733–1738. doi: 10.1007/s00604-015-1506-8. - DOI
-
- Yu Y., Ju P., Zhang D., Han X., Yin X., Zheng L., Sun C. Peroxidase-like activity of FeVO4 nanobelts and its analytical application for optical detection of hydrogen peroxide. Sens. Actuators B Chem. 2016;233:162–172. doi: 10.1016/j.snb.2016.04.041. - DOI
-
- Qiao F., Chen L., Li X., Li L., Ai S. Peroxidase-like activity of manganese selenide nanoparticles and its analytical application for visual detection of hydrogen peroxide and glucose. Sens. Actuators B Chem. 2014;193:255–262. doi: 10.1016/j.snb.2013.11.108. - DOI
-
- Yang Z., Ji H. 2-Hydroxypropyl-β-cyclodextrin polymer as a mimetic enzyme for mediated synthesis of benzaldehyde in water. ACS. Sustain. Chem. Eng. 2013;1:1172–1179. doi: 10.1021/sc4001059. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

