Bone marrow-derived mesenchymal stem/stromal cells reverse the sensorial diabetic neuropathy via modulation of spinal neuroinflammatory cascades
- PMID: 29933760
- PMCID: PMC6015468
- DOI: 10.1186/s12974-018-1224-3
Bone marrow-derived mesenchymal stem/stromal cells reverse the sensorial diabetic neuropathy via modulation of spinal neuroinflammatory cascades
Abstract
Background: Diabetic neuropathy (DN) is a frequent and debilitating manifestation of diabetes mellitus, to which there are no effective therapeutic approaches. Mesenchymal stem/stromal cells (MSC) have a great potential for the treatment of this syndrome, possibly through regenerative actions on peripheral nerves. Here, we evaluated the therapeutic effects of MSC on spinal neuroinflammation, as well as on ultrastructural aspects of the peripheral nerve in DN-associated sensorial dysfunction.
Methods: C57Bl/6 mice were treated with bone marrow-derived MSC (1 × 106), conditioned medium from MSC cultures (CM-MSC) or vehicle by endovenous route following the onset of streptozotocin (STZ)-induced diabetes. Paw mechanical and thermal nociceptive thresholds were evaluated by using von Frey filaments and Hargreaves test, respectively. Morphological and morphometric analysis of the sciatic nerve was performed by light microscopy and transmission electron microscopy. Mediators and markers of neuroinflammation in the spinal cord were measured by radioimmunoassay, real-time PCR, and immunofluorescence analyses.
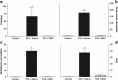
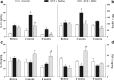
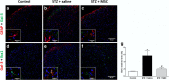
Results: Diabetic mice presented behavioral signs of sensory neuropathy, mechanical allodynia, and heat hypoalgesia, which were completely reversed by a single administration of MSC or CM-MSC. The ultrastructural analysis of the sciatic nerve showed that diabetic mice exhibited morphological and morphometric alterations, considered hallmarks of DN, such as degenerative changes in axons and myelin sheath, and reduced area and density of unmyelinated fibers. In MSC-treated mice, these structural alterations were markedly less commonly observed and/or less pronounced. Moreover, MSC transplantation inhibited multiple parameters of spinal neuroinflammation found in diabetic mice, causing the reduction of activated astrocytes and microglia, oxidative stress signals, galectin-3, IL-1β, and TNF-α production. Conversely, MSC increased the levels of anti-inflammatory cytokines, IL-10, and TGF-β.
Conclusions: The present study described the modulatory effects of MSC on spinal cord neuroinflammation in diabetic mice, suggesting new mechanisms by which MSC can improve DN.
Keywords: Diabetes; Galectin-3; Neuroinflammation; Sensory neuropathy; Spinal cord; Stem cells.
Conflict of interest statement
Ethics approval and consent to participate
Animal care and handling procedures were in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH, 8023) and the Institutional Animal Care and Use Committee FIOCRUZ (CPqGM 025/2011).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

