Use of Air-activated Thermal Devices during Recovery after Surgery in Mice
- PMID: 29933764
- PMCID: PMC6059220
- DOI: 10.30802/AALAS-JAALAS-17-000077
Use of Air-activated Thermal Devices during Recovery after Surgery in Mice
Abstract
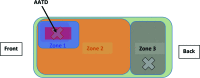
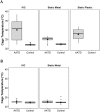
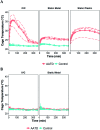
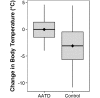
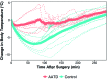
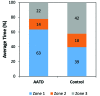
Laboratory mice (Mus musculus) are susceptible to hypothermia, especially during anesthetic events, disease states, and exposure to environmental stressors. Thermal support devices for small mammals are numerous, but often require a power source and may be impractical to use for cages on a rack. Air-activated thermal devices (AATD) are mixtures of chemicals that cause an exothermic reaction. In this study, we examined the environmental effects of AATD on internal cage temperatures without the use of additional equipment as well as the physiologic effects of AATD as postoperative thermal support in mice. For environmental experiments, temperatures measured inside the cage and above the AATD peaked at 35.6 ± 2.5 °C (13.4 °C higher than control cages). We also demonstrated that the amount of heat produced by AATD and its temporal distribution are dependent on cage and rack types. For physiologic experiments, mice were surgically implanted with an intraperitoneal temperature telemetry device in a static cage setting. Recovery times and final body temperature at 5 h postoperatively did not differ significantly between mice with and without AATD. During the first 0 to 3 h after mice returned to their home cages, body temperature dropped markedly in mice without AATD but not in mice with AATD. Based on this result the physiologic results of our study support that AATD can be useful in providing extended thermal support for mice housed in static microisolation cages to help maintain body temperature postsurgically. Environmental results of our studies demonstrated that AATD provide local clinically relevant thermal support for 2.5 to 6 h, depending on cage set-up.
Figures

References
-
- Baran S, Flegal M, Johnson E, Perret-gentil M. [Internet]. 2017. Intraoperative physiological monitoring in rodent surgical research. [Cited 11 June 2018]. Available at: https://www.alnmag.com/article/2012/10/intraoperative-physiological-moni...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

