Disulfide-crosslink scanning reveals prion-induced conformational changes and prion strain-specific structures of the pathological prion protein PrPSc
- PMID: 29934306
- PMCID: PMC6102138
- DOI: 10.1074/jbc.RA117.001633
Disulfide-crosslink scanning reveals prion-induced conformational changes and prion strain-specific structures of the pathological prion protein PrPSc
Erratum in
-
Correction: Disulfide-crosslink scanning reveals prion-induced conformational changes and prion strain-specific structures of the pathological prion protein PrPSc.J Biol Chem. 2018 Sep 21;293(38):14925. doi: 10.1074/jbc.AAC118.005526. J Biol Chem. 2018. PMID: 30242041 Free PMC article. No abstract available.
Abstract
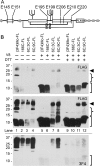
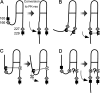
Prions are composed solely of the pathological isoform (PrPSc) of the normal cellular prion protein (PrPC). Identification of different PrPSc structures is crucially important for understanding prion biology because the pathogenic properties of prions are hypothesized to be encoded in the structures of PrPSc However, these structures remain yet to be identified, because of the incompatibility of PrPSc with conventional high-resolution structural analysis methods. Previously, we reported that the region between the first and the second α-helix (H1∼H2) of PrPC might cooperate with the more C-terminal side region for efficient interactions with PrPSc From this starting point, we created a series of PrP variants with two cysteine substitutions (C;C-PrP) forming a disulfide-crosslink between H1∼H2 and the distal region of the third helix (Ctrm). We then assessed the conversion capabilities of the C;C-PrP variants in N2a cells infected with mouse-adapted scrapie prions (22L-ScN2a). Specifically, Cys substitutions at residues 165, 166, or 168 in H1∼H2 were combined with cysteine scanning along Ctrm residues 220-229. We found that C;C-PrPs are expressed normally with glycosylation patterns and subcellular localization similar to WT PrP, albeit differing in expression levels. Interestingly, some C;C-PrPs converted to protease-resistant isoforms in the 22L-ScN2a cells, but not in Fukuoka1 prion-infected cells. Crosslink patterns of convertible C;C-PrPs indicated a positional change of H1∼H2 toward Ctrm in PrPSc-induced conformational conversion. Given the properties of the C;C-PrPs reported here, we propose that these PrP variants may be useful tools for investigating prion strain-specific structures and structure-phenotype relationships of PrPSc.
Keywords: prion; prion conversion; prion disease; prion protein; protein conformation; protein crosslinking; protein misfolding; protein structure.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




Similar articles
-
Strain-Dependent Prion Infection in Mice Expressing Prion Protein with Deletion of Central Residues 91-106.Int J Mol Sci. 2020 Oct 1;21(19):7260. doi: 10.3390/ijms21197260. Int J Mol Sci. 2020. PMID: 33019549 Free PMC article.
-
Prion Protein Devoid of the Octapeptide Repeat Region Delays Bovine Spongiform Encephalopathy Pathogenesis in Mice.J Virol. 2017 Dec 14;92(1):e01368-17. doi: 10.1128/JVI.01368-17. Print 2018 Jan 1. J Virol. 2017. PMID: 29046443 Free PMC article.
-
The charge structure of helix 1 in the prion protein regulates conversion to pathogenic PrPSc.J Virol. 2006 Sep;80(17):8521-9. doi: 10.1128/JVI.00366-06. J Virol. 2006. PMID: 16912302 Free PMC article.
-
Transition of the prion protein from a structured cellular form (PrPC ) to the infectious scrapie agent (PrPSc ).Protein Sci. 2019 Dec;28(12):2055-2063. doi: 10.1002/pro.3735. Epub 2019 Oct 25. Protein Sci. 2019. PMID: 31583788 Free PMC article. Review.
-
Evolving views in prion glycosylation: functional and pathological implications.Biochimie. 2003 Jan-Feb;85(1-2):33-45. doi: 10.1016/s0300-9084(03)00040-3. Biochimie. 2003. PMID: 12765773 Review.
Cited by
-
New developments in prion disease research.Cell Tissue Res. 2023 Apr;392(1):1-5. doi: 10.1007/s00441-023-03760-y. Cell Tissue Res. 2023. PMID: 36918429 No abstract available.
-
Systematic conformation-to-phenotype mapping via limited deep-sequencing of proteins.ArXiv [Preprint]. 2023 Jan 30:arXiv:2204.06159v2. ArXiv. 2023. Update in: Mol Cell. 2023 Jun 1;83(11):1936-1952.e7. doi: 10.1016/j.molcel.2023.05.006. PMID: 36776823 Free PMC article. Updated. Preprint.
-
Mechanisms of Strain Diversity of Disease-Associated in-Register Parallel β-Sheet Amyloids and Implications About Prion Strains.Viruses. 2019 Jan 28;11(2):110. doi: 10.3390/v11020110. Viruses. 2019. PMID: 30696005 Free PMC article. Review.
-
Systematic conformation-to-phenotype mapping via limited deep sequencing of proteins.Mol Cell. 2023 Jun 1;83(11):1936-1952.e7. doi: 10.1016/j.molcel.2023.05.006. Mol Cell. 2023. PMID: 37267908 Free PMC article.
-
Conformation-Dependent Influences of Hydrophobic Amino Acids in Two In-Register Parallel β-Sheet Amyloids, an α-Synuclein Amyloid and a Local Structural Model of PrPSc.ACS Omega. 2022 Aug 24;7(35):31271-31288. doi: 10.1021/acsomega.2c03523. eCollection 2022 Sep 6. ACS Omega. 2022. PMID: 36092583 Free PMC article.
References
-
- Telling G. C., Parchi P., DeArmond S. J., Cortelli P., Montagna P., Gabizon R., Mastrianni J., Lugaresi E., Gambetti P., and Prusiner S. B. (1996) Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274, 2079–2082 10.1126/science.274.5295.2079 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

