A potent, proteolysis-resistant inhibitor of kallikrein-related peptidase 6 (KLK6) for cancer therapy, developed by combinatorial engineering
- PMID: 29934309
- PMCID: PMC6102146
- DOI: 10.1074/jbc.RA117.000871
A potent, proteolysis-resistant inhibitor of kallikrein-related peptidase 6 (KLK6) for cancer therapy, developed by combinatorial engineering
Abstract
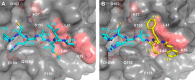
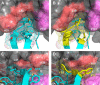
Human tissue kallikrein (KLK) proteases are hormone-like signaling molecules with important functions in cancer pathophysiology. KLK-related peptidase 6 (KLK6), specifically, is highly up-regulated in several types of cancer, where its increased activity promotes cancer invasion and metastasis. This characteristic suggests KLK6 as an attractive target for therapeutic interventions. However, inhibitors that specifically target KLK6 have not yet been reported, possibly because KLK6 shares a high sequence homology and structural similarity with other serine proteases and resists inhibition by many polypeptide inhibitors. Here, we present an innovative combinatorial approach to engineering KLK6 inhibitors via flow cytometry-based screening of a yeast-displayed mutant library of the human amyloid precursor protein Kunitz protease inhibitor domain (APPI), an inhibitor of other serine proteases, such as anionic and cationic trypsins. On the basis of this screening, we generated APPIM17L,I18F,S19F,F34V (APPI-4M), an APPI variant with a KLK6 inhibition constant (Ki ) of 160 pm and a turnover time of 10 days. To the best of our knowledge, APPI-4M is the most potent KLK6 inhibitor reported to date, displaying 146-fold improved affinity and 13-fold improved proteolytic stability compared with WT APPI (APPIWT). We further demonstrate that APPI-4M acts as a functional inhibitor in a cell-based model of KLK6-dependent breast cancer invasion. Finally, the crystal structures of the APPIWT/KLK6 and APPI-4M/KLK6 complexes revealed the structural and mechanistic bases for the improved KLK6 binding and proteolytic resistance of APPI-4M. We anticipate that APPI-4M will have substantial translational potential as both imaging agent and therapeutic.
Keywords: X-ray crystallography; cell invasion; directed evolution; enzyme inhibition; protease inhibitor; protein engineering; proteolysis; selective binding; yeast surface display.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
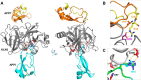
References
-
- Oikonomopoulou K., Diamandis E. P., and Hollenberg M. D. (2010) Kallikrein-related peptidases: proteolysis and signaling in cancer, the new frontier. Biol. Chem. 391, 299–310 - PubMed
-
- Kontos C. K., and Scorilas A. (2012) Kallikrein-related peptidases (KLKs): a gene family of novel cancer biomarkers. Clin. Chem. Lab. Med. 50, 1877–1891 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

