Nitrogen Addition Decreases Dissimilatory Nitrate Reduction to Ammonium in Rice Paddies
- PMID: 29934331
- PMCID: PMC6102975
- DOI: 10.1128/AEM.00870-18
Nitrogen Addition Decreases Dissimilatory Nitrate Reduction to Ammonium in Rice Paddies
Abstract
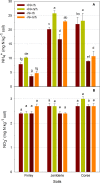
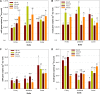
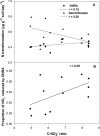
Dissimilatory nitrate reduction to ammonium (DNRA), denitrification, anaerobic ammonium oxidation (anammox), and biological N2 fixation (BNF) can influence the nitrogen (N) use efficiency of rice production. While the effect of N application on BNF is known, little is known about its effect on NO3- partitioning between DNRA, denitrification, and anammox. Here, we investigated the effect of N application on DNRA, denitrification, anammox, and BNF and on the abundance of relevant genes in three paddy soils in Australia. Rice was grown in a glasshouse with N fertilizer (150 kg N ha-1) and without N fertilizer for 75 days, and the rhizosphere and bulk soils were collected separately for laboratory incubation and quantitative PCR analysis. Nitrogen application reduced DNRA rates by >16% in all the soils regardless of the rhizospheric zone, but it did not affect the nrfA gene abundance. Without N, the amount and proportion of NO3- reduced by DNRA (0.42 to 0.52 μg g-1 soil day-1 and 45 to 55%, respectively) were similar to or higher than the amount and proportion reduced by denitrification. However, with N the amount of NO3- reduced by DNRA (0.32 to 0.40 μg g-1 soil day-1) was 40 to 50% lower than the amount of NO3- reduced by denitrification. Denitrification loss increased by >20% with N addition and was affected by the rhizospheric zones. Nitrogen loss was minimal through anammox, while BNF added 0.02 to 0.25 μg N g-1 soil day-1 We found that DNRA plays a significant positive role in paddy soil N retention, as it accounts for up to 55% of the total NO3- reduction, but this is reduced by N application.IMPORTANCE This study provides evidence that nitrogen addition reduces nitrogen retention through DNRA and increases nitrogen loss via denitrification in a paddy soil ecosystem. DNRA is one of the major NO3- reduction processes, and it can outcompete denitrification in NO3- consumption when rice paddies are low in nitrogen. A significant level of DNRA activity in paddy soils indicates that DNRA plays an important role in retaining nitrogen by reducing NO3- availability for denitrification and leaching. Our study shows that by reducing N addition to rice paddies, there is a positive effect from reduced nitrogen loss but, more importantly, from the conversion of NO3- to NH4+, which is the favored form of mineral nitrogen for plant uptake.
Keywords: denitrification; dissimilatory nitrate reduction to ammonium; nitrogen; nrfA; rice paddies.
Copyright © 2018 American Society for Microbiology.
Figures

References
-
- Heffer P. 2013. Assessment of fertilizer use by crop at the global level. International Fertilizer Industry Association, Paris, France: https://www.fertilizer.org/images/Library_Downloads/AgCom.13.39%20-%20FU....
-
- Ussiri D, Lal R. 2013. Nitrous oxide emissions from rice fields, p 213–242. In Soil emission of nitrous oxide and its mitigation. Springer, Dordrecht, Netherlands.
-
- Kögel-Knabner I, Amelung W, Cao Z, Fiedler S, Frenzel P, Jahn R, Kalbitz K, Kölbl A, Schloter M. 2010. Biogeochemistry of paddy soils. Geoderma 157:1–14. doi: 10.1016/j.geoderma.2010.03.009. - DOI
-
- Li YL, Fan XR, Shen QR. 2008. The relationship between rhizosphere nitrification and nitrogen-use efficiency in rice plants. Plant Cell Environ 31:73–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

