Heart Failure After Ischemic Stroke or Transient Ischemic Attack in Insulin-Resistant Patients Without Diabetes Mellitus Treated With Pioglitazone
- PMID: 29934374
- PMCID: PMC6202153
- DOI: 10.1161/CIRCULATIONAHA.118.034763
Heart Failure After Ischemic Stroke or Transient Ischemic Attack in Insulin-Resistant Patients Without Diabetes Mellitus Treated With Pioglitazone
Abstract
Background: The IRIS trial (Insulin Resistance Intervention After Stroke) demonstrated that pioglitazone reduced the risk for both cardiovascular events and diabetes mellitus in insulin-resistant patients. However, concern remains that pioglitazone may increase the risk for heart failure (HF) in susceptible individuals.
Methods: In IRIS, patients with insulin resistance but without diabetes mellitus were randomized to pioglitazone or placebo (1:1) within 180 days of an ischemic stroke or transient ischemic attack and followed for ≤5 years. To identify patients at higher HF risk with pioglitazone, we performed a secondary analysis of IRIS participants without HF history at entry. HF episodes were adjudicated by an external review, and treatment effects were analyzed using time-to-event methods. A baseline HF risk score was constructed from a Cox model estimated using stepwise selection. Baseline patient features (individually and summarized in risk score) and postrandomization events were examined as possible modifiers of the effect of pioglitazone. Net cardiovascular benefit was estimated for the composite of stroke, myocardial infarction, and hospitalized HF.
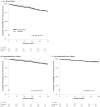
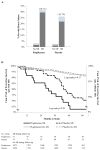
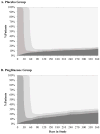
Results: Among 3851 patients, the mean age was 63 years, and 65% were male. The 5-year HF risk did not differ by treatment (4.1% pioglitazone, 4.2% placebo). Risk for hospitalized HF was low and not significantly greater in pioglitazone compared with placebo groups (2.9% versus 2.3%, P=0.36). Older age, atrial fibrillation, hypertension, obesity, edema, high C-reactive protein, and smoking were risk factors for HF. However, the effect of pioglitazone did not differ across levels of baseline HF risk (hazard ratio [95% CI] for pioglitazone versus placebo for patients at low, moderate, and high risk: 1.03 [0.61-1.73], 1.10 [0.56-2.15], and 1.08 [0.58-2.01]; interaction P value=0.98). HF risk was increased in patients with versus those without incident myocardial infarction in both groups (pioglitazone: 31.4% versus 2.7%; placebo: 25.7% versus 2.4%; P<0.0001). Edema, dyspnea, and weight gain in the trial did not predict HF hospitalization but led to more study drug dose reduction with a lower mean dose of pioglitazone versus placebo (29±17 mg versus 33±15 mg, P<0.0001). Pioglitazone reduced the composite outcome of stroke, myocardial infarction, or hospitalized HF (hazard ratio, 0.78; P=0.007).
Conclusions: In IRIS, with surveillance and dose adjustments, pioglitazone did not increase the risk of HF and conferred net cardiovascular benefit in patients with insulin resistance and cerebrovascular disease. The risk of HF with pioglitazone was not modified by baseline HF risk. The IRIS experience may be instructive for maximizing the net benefit of this therapy.
Clinical trial registration: URL: https://www.clinicaltrials.gov . Unique identifier: NCT00091949.
Keywords: heart failure; insulin resistance; pioglitazone; stroke; thiazolidinedione.
Figures
Comment in
-
Pioglitazone Use After Stroke: Story of Hearts, Minds, and Bones.Circulation. 2018 Sep 18;138(12):1221-1223. doi: 10.1161/CIRCULATIONAHA.118.036147. Circulation. 2018. PMID: 30354440 No abstract available.
References
-
- Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754–1759. - PubMed
-
- Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, Brass LM, Schwartz GG, Adams HP, Jr, Berger L, Carolei A, Clark W, Coull B, Ford GA, Kleindorfer D, O’Leary JR, Parsons MW, Ringleb P, Sen S, Spence JD, Tanne D, Wang D, Winder T for the IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–1331. - PMC - PubMed
-
- van der Meer RW, Rijzewijk LJ, de Jong HWAM, Lamb HJ, Lubberink M, Romijn JA, Bax JJ, de Roos A, Kamp O, Paulus WJ, Heine RJ, Lammertsma AA, Smit JWA, Diamant M. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation. 2009;119:2069–2077. - PubMed
-
- Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association - October 7, 2003. Circulation. 2003;108:2941–2948. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

