Clinical Pharmacokinetics in Kidney Disease: Fundamental Principles
- PMID: 29934432
- PMCID: PMC6032582
- DOI: 10.2215/CJN.00340118
Clinical Pharmacokinetics in Kidney Disease: Fundamental Principles
Abstract
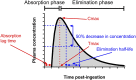
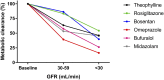
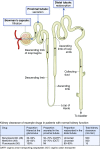
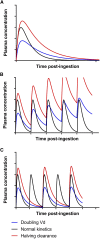
Kidney disease is an increasingly common comorbidity that alters the pharmacokinetics of many drugs. Prescribing to patients with kidney disease requires knowledge about the drug, the extent of the patient's altered physiology, and pharmacokinetic principles that influence the design of dosing regimens. There are multiple physiologic effects of impaired kidney function, and the extent to which they occur in an individual at any given time can be difficult to define. Although some guidelines are available for dosing in kidney disease, they may be on the basis of limited data or not widely applicable, and therefore, an understanding of pharmacokinetic principles and how to apply them is important to the practicing clinician. Whether kidney disease is acute or chronic, drug clearance decreases, and the volume of distribution may remain the same or increase. Although in CKD, these changes progress relatively slowly, they are dynamic in AKI, and recovery is possible depending on the etiology and treatments. This, and the use of kidney replacement therapies further complicate attempts to quantify drug clearance at the time of prescribing and dosing in AKI. The required change in the dosing regimen can be estimated or even quantitated in certain instances through the application of pharmacokinetic principles to guide rational drug dosing. This offers an opportunity to provide personalized medical care and minimizes adverse drug events from either under- or overdosing. We discuss the principles of pharmacokinetics that are fundamental for the design of an appropriate dosing regimen in this review.
Keywords: acute kidney injury; chronic kidney disease; clearance; dialysis; kidney disease; pharmacokinetics; volume of distribution.
Copyright © 2018 by the American Society of Nephrology.
Figures

References
-
- Zhang Y, Zhang L, Abraham S, Apparaju S, Wu TC, Strong JM, Xiao S, Atkinson AJ Jr, Thummel KE, Leeder JS, Lee C, Burckart GJ, Lesko LJ, Huang SM: Assessment of the impact of renal impairment on systemic exposure of new molecular entities: Evaluation of recent new drug applications. Clin Pharmacol Ther 85: 305–311, 2009 - PubMed
-
- FDA: Pharmacokinetics in Patients with Impaired Renal Function—Study Design, Data Analysis, and Impact on Dosing and Labeling, 2010. Available at: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformat.... Accessed December 24, 2017
-
- Khanal A, Castelino RL, Peterson GM, Jose MD: Dose adjustment guidelines for medications in patients with renal impairment: How consistent are drug information sources? Intern Med J 44: 77–85, 2014 - PubMed
-
- Duong JK, Roberts DM, Furlong TJ, Kumar SS, Greenfield JR, Kirkpatrick CM, Graham GG, Williams KM, Day RO: Metformin therapy in patients with chronic kidney disease. Diabetes Obes Metab 14: 963–965, 2012 - PubMed
-
- Leil TA, Feng Y, Zhang L, Paccaly A, Mohan P, Pfister M: Quantification of apixaban’s therapeutic utility in prevention of venous thromboembolism: Selection of phase III trial dose. Clin Pharmacol Ther 88: 375–382, 2010 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

