Lactobacillus rhamnosus GG protects the intestinal epithelium from radiation injury through release of lipoteichoic acid, macrophage activation and the migration of mesenchymal stem cells
- PMID: 29934438
- PMCID: PMC7202371
- DOI: 10.1136/gutjnl-2018-316226
Lactobacillus rhamnosus GG protects the intestinal epithelium from radiation injury through release of lipoteichoic acid, macrophage activation and the migration of mesenchymal stem cells
Abstract
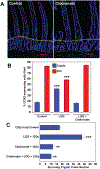
Objective: Lactobacillus rhamnosus GG (LGG), a probiotic, given by gavage is radioprotective of the mouse intestine. LGG-induced radioprotection is toll-like receptor 2 (TLR2) and cyclooxygenase-2 (COX-2)-dependent and is associated with the migration of COX-2+mesenchymal stem cells (MSCs) from the lamina propria of the villus to the lamina propria near the crypt epithelial stem cells. Our goals were to define the mechanism of LGG radioprotection including identification of the TLR2 agonist, and the mechanism of the MSC migration and to determine the safety and efficacy of this approach in models relevant to clinical radiation therapy.
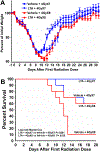
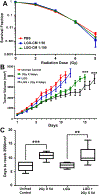
Design: Intestinal radioprotection was modelled in vitro with cell lines and enteroids as well as in vivo by assaying clinical outcomes and crypt survival. Fractionated abdominal and single dose radiation were used along with syngeneic CT26 colon tumour grafts to assess tumour radioprotection.
Results: LGG with a mutation in the processing of lipoteichoic acid (LTA), a TLR2 agonist, was not radioprotective, while LTA agonist and native LGG were. An agonist of CXCR4 blocked LGG-induced MSC migration and LGG-induced radioprotection. LGG given by gavage induced expression of CXCL12, a CXCR4 agonist, in pericryptal macrophages and depletion of macrophages by clodronate liposomes blocked LGG-induced MSC migration and radioprotection. LTA effectively protected the normal intestinal crypt, but not tumours in fractionated radiation regimens.
Conclusions: LGG acts as a 'time-release capsule' releasing radioprotective LTA. LTA then primes the epithelial stem cell niche to protect epithelial stem cells by triggering a multicellular, adaptive immune signalling cascade involving macrophages and PGE2 secreting MSCs.
Trial registration number: NCT01790035; Pre-results.
Keywords: intestinal epithelium; probiotics; prostaglandins; radiotherapy; stem cells.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2019. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures




References
-
- Potten CS. A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiat Biol 1990;58:925–73. - PubMed
-
- Bismar MM, Sinicrope FA. Radiation enteritis. Current Gastroenterology Reports 2002;4:361–5. - PubMed
-
- Wardill HR, Van Sebille YZA, Ciorba MA, Bowen JM. Prophylactic probiotics for cancer therapy-induced diarrhoea: a meta-analysis. Current opinion in supportive and palliative care 2018;12:187–97. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
