Uterine glands coordinate on-time embryo implantation and impact endometrial decidualization for pregnancy success
- PMID: 29934619
- PMCID: PMC6015089
- DOI: 10.1038/s41467-018-04848-8
Uterine glands coordinate on-time embryo implantation and impact endometrial decidualization for pregnancy success
Abstract
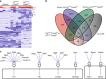
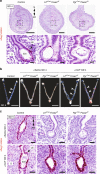
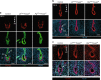
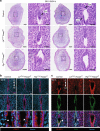
Uterine glands are essential for pregnancy establishment. By employing forkhead box A2 (FOXA2)-deficient mouse models coupled with leukemia inhibitory factor (LIF) repletion, we reveal definitive roles of uterine glands in embryo implantation and stromal cell decidualization. Here we report that LIF from the uterine glands initiates embryo-uterine communication, leading to embryo attachment and stromal cell decidualization. Detailed histological and molecular analyses discovered that implantation crypt formation does not involve uterine glands, but removal of the luminal epithelium is delayed and subsequent decidualization fails in LIF-replaced glandless but not gland-containing FOXA2-deficient mice. Adverse ripple effects of those dysregulated events in the glandless uterus result in embryo resorption and pregnancy failure. These studies provide evidence that uterine glands synchronize embryo-endometrial interactions, coordinate on-time embryo implantation, and impact stromal cell decidualization, thereby ensuring embryo viability, placental growth, and pregnancy success.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD096266/HD/NICHD NIH HHS/United States
- R21 HD076347/HD/NICHD NIH HHS/United States
- R21HD076347/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/International
- R01HD096266/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

