Diabetic Gastroparesis: Principles and Current Trends in Management
- PMID: 29934758
- PMCID: PMC6028327
- DOI: 10.1007/s13300-018-0454-9
Diabetic Gastroparesis: Principles and Current Trends in Management
Abstract
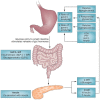
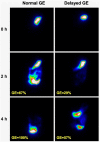
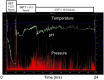
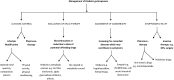
This article is a comprehensive review of diabetic gastroparesis, defined as delayed or disordered gastric emptying, including basic principles and current trends in management. This review includes sections on anatomy and physiology, diagnosis and differential diagnosis as well as management and current guidelines for treatment of diabetic gastroparesis. Diabetic gastroparesis (DGp) is a component of autonomic neuropathy resulting from long-standing poorly controlled type 1 and type 2 diabetes. The diagnostic workup of DGp first excludes obstruction and other causes including medications that may mimic delayed/disordered gastric emptying. Targeting nutrition, hydration, symptomatic relief and glycemic control are mainstays of treatment for DGp. Additionally, optimal treatment of DGp includes good glycemic management, often involving customizing insulin delivery using basal-bolus insulin and technology, including sensor-augmented pumps and continuous glucose monitoring systems. Prokinetic medications may be helpful in DGp symptoms, although only limited number of medications is currently available in the USA. Selected medication-refractory patients with DGp may benefit from gastric neuromodulation, and some from surgical interventions including pyloric therapies that can also be done endoscopically. As is true of any of the diabetic complications, prevention of DGp by early and optimal glycemic control is more cost-effective.Funding: Hansa Medcell, India.
Keywords: Diabetes; Gastroparesis; Glucose; Insulin nausea; Type 1 diabetes; Type 2 diabetes; Vomiting.
Figures
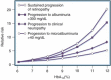
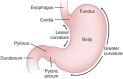
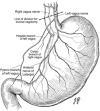
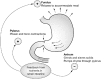

References
-
- Kassander P. Asymptomatic gastric retention in diabetics (gastroparesis diabeticorum) Ann Intern Med. 1958;48:797–812. - PubMed
-
- Horowitz M, Harding PE, Maddox AF, et al. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:151–159. - PubMed
-
- Bekele G, Kabadi UM. Gastro intestinal manifestations of diabetes mellitus. Int J Diabetes Dev Countries. 1996;16:54–58.
-
- Ajumobi AB, Griffin RA. Diabetic gastroparesis: evaluation and management. Hosp Physician. 2008;44:27–35.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

