Alzheimer's disease pathology propagation by exosomes containing toxic amyloid-beta oligomers
- PMID: 29934873
- PMCID: PMC6015111
- DOI: 10.1007/s00401-018-1868-1
Alzheimer's disease pathology propagation by exosomes containing toxic amyloid-beta oligomers
Abstract
The gradual deterioration of cognitive functions in Alzheimer's disease is paralleled by a hierarchical progression of amyloid-beta and tau brain pathology. Recent findings indicate that toxic oligomers of amyloid-beta may cause propagation of pathology in a prion-like manner, although the underlying mechanisms are incompletely understood. Here we show that small extracellular vesicles, exosomes, from Alzheimer patients' brains contain increased levels of amyloid-beta oligomers and can act as vehicles for the neuron-to-neuron transfer of such toxic species in recipient neurons in culture. Moreover, blocking the formation, secretion or uptake of exosomes was found to reduce both the spread of oligomers and the related toxicity. Taken together, our results imply that exosomes are centrally involved in Alzheimer's disease and that they could serve as targets for development of new diagnostic and therapeutic principles.
Keywords: Alzheimer’s disease; Beta-amyloid; Exosomes; Human; Oligomers; Prion-like; Propagation.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
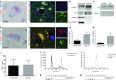
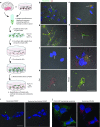
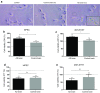
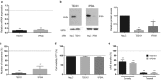
Comment in
-
Exosomes can spread toxic AD pathology.Nat Rev Neurol. 2018 Aug;14(8):451. doi: 10.1038/s41582-018-0039-2. Nat Rev Neurol. 2018. PMID: 29934584 No abstract available.
References
-
- Agholme L, Nath S, Domert J, Marcusson J, Kagedal K, Hallbeck M. Proteasome inhibition induces stress kinase dependent transport deficits—implications for Alzheimer’s disease. Mol Cell Neurosci. 2013;58C:29–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

