Does growth impairment underlie the adverse effects of dexamethasone on development of noradrenergic systems?
- PMID: 29935188
- PMCID: PMC6150779
- DOI: 10.1016/j.tox.2018.06.008
Does growth impairment underlie the adverse effects of dexamethasone on development of noradrenergic systems?
Abstract
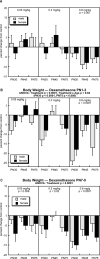
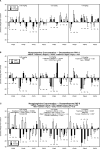
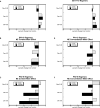
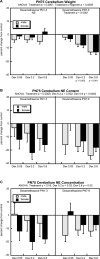
Glucocorticoids are given in preterm labor to prevent respiratory distress but these agents evoke neurobehavioral deficits in association with reduced brain region volumes. To determine whether the neurodevelopmental effects are distinct from growth impairment, we gave developing rats dexamethasone at doses below or within the therapeutic range (0.05, 0.2 or 0.8 mg/kg) at different stages: gestational days (GD) 17-19, postnatal days (PN) 1-3 or PN7-9. In adolescence and adulthood, we assessed the impact on noradrenergic systems in multiple brain regions, comparing the effects to those on somatic growth or on brain region growth. Somatic growth was reduced with exposure in all three stages, with greater sensitivity for the postnatal regimens; brain region growth was impaired to a lesser extent. Norepinephrine content and concentration were reduced depending on the treatment regimen, with a rank order of deficits of PN7-9 > PN1-3 > GD17-19. However, brain growth impairment did not parallel reduced norepinephrine content in magnitude, dose threshold, sex or regional selectivity, or temporal pattern, and even when corrected for reduced brain region weights (norepinephrine per g tissue), the dexamethasone-exposed animals showed subnormal values. Regression analysis showed that somatic growth impairment accounted for an insubstantial amount of the reduction in norepinephrine content, and brain growth impairment accounted for only 12%, whereas specific effects on norepinephrine accounted for most of the effect. The adverse effects of dexamethasone on noradrenergic system development are not simply related to impaired somatic or brain region growth, but rather include specific targeting of neurodifferentiation.
Keywords: Dexamethasone; Growth impairment; Norepinephrine; Preterm labor.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures




Similar articles
-
Prenatal drug exposures sensitize noradrenergic circuits to subsequent disruption by chlorpyrifos.Toxicology. 2015 Dec 2;338:8-16. doi: 10.1016/j.tox.2015.09.005. Epub 2015 Sep 28. Toxicology. 2015. PMID: 26419632 Free PMC article.
-
Mimicking maternal smoking and pharmacotherapy of preterm labor: fetal nicotine exposure enhances the effect of late gestational dexamethasone treatment on noradrenergic circuits.Brain Res Bull. 2011 Nov 25;86(5-6):435-40. doi: 10.1016/j.brainresbull.2011.08.009. Epub 2011 Aug 19. Brain Res Bull. 2011. PMID: 21875656 Free PMC article.
-
Fetal dexamethasone exposure interferes with establishment of cardiac noradrenergic innervation and sympathetic activity.Teratology. 1993 Feb;47(2):109-17. doi: 10.1002/tera.1420470203. Teratology. 1993. PMID: 8446924
-
Neurotoxicity of glucocorticoids in the primate brain.Horm Behav. 1994 Dec;28(4):336-48. doi: 10.1006/hbeh.1994.1030. Horm Behav. 1994. PMID: 7729802 Review.
-
Selective Lifelong Destruction of Brain Monoaminergic Nerves Through Perinatal DSP-4 Treatment.Curr Top Behav Neurosci. 2016;29:51-71. doi: 10.1007/7854_2015_398. Curr Top Behav Neurosci. 2016. PMID: 26427851 Review.
Cited by
-
MSC Based Therapies to Prevent or Treat BPD-A Narrative Review on Advances and Ongoing Challenges.Int J Mol Sci. 2021 Jan 24;22(3):1138. doi: 10.3390/ijms22031138. Int J Mol Sci. 2021. PMID: 33498887 Free PMC article. Review.
References
-
- Cavalieri RL, Cohen WR. Antenatal steroid therapy: have we undervalued the risks? J Matern Fetal Neonat Med. 2006;19:265–269. - PubMed
-
- Cheong JLY, Burnett AC, Lee KJ, Roberts G, Thompson DK, Wood SJ, Connelly A, Anderson PJ, Doyle LW, Victorian Infant Collaborative Study Group Association between postnatal dexamethasone for treatment of bronchopulmonary dysplasia and brain volumes at adolescence in infants born very preterm. J Pediatr. 2014;164:737–743. - PMC - PubMed
-
- Ciaranello RD, Jacobowitz D, Axelrod J. Effect of dexamethasone on phenylethanolamine N-methyltransferase in chromaffin tissue of the neonatal rat. J Neurochem. 1973;20:799–805. - PubMed
-
- Crowther CA, Doyle LW, Haslam RR, Hiller JE, Harding JE, Robinson J, Actords Study Group Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. New Eng J Med. 2007;357:1179–1189. - PubMed
-
- Dammann O, Matthews SG. Repeated antenatal glucocorticoid exposure and the developing brain. Pediatr Res. 2001;50:563–564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

