Expression of cytokines and chemokines in mouse skin treated with sulfur mustard
- PMID: 29935281
- PMCID: PMC6438172
- DOI: 10.1016/j.taap.2018.06.008
Expression of cytokines and chemokines in mouse skin treated with sulfur mustard
Abstract
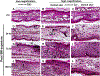
Sulfur mustard (2,2'-dichlorodiethyl sulfide, SM) is a chemical warfare agent that generates an inflammatory response in the skin and causes severe tissue damage and blistering. In earlier studies, we identified cutaneous damage induced by SM in mouse ear skin including edema, erythema, epidermal hyperplasia and microblistering. The present work was focused on determining if SM-induced injury was associated with alterations in mRNA and protein expression of specific cytokines and chemokines in the ear skin. We found that SM caused an accumulation of macrophages and neutrophils in the tissue within one day which persisted for at least 7 days. This was associated with a 2-15 fold increase in expression of the proinflammatory cytokines interleukin-1β, interleukin-6, and tumor necrosis factor α at time points up to 7 days post-SM exposure. Marked increases (20-1000 fold) in expression of chemokines associated with recruitment and activation of macrophages were also noted in the tissue including growth-regulated oncogene α (GROα/CXCL1), monocyte chemoattractant protein 1 (MCP-1/CCL2), granulocyte-colony stimulating factor (GCSF/CSF3), macrophage inflammatory protein 1α (MIP1α/CCL3), and IFN-γ-inducible protein 10 (IP10/CXCL10). The pattern of cytokines/chemokine expression was coordinate with expression of macrophage elastase/MMP12 and neutrophil collagenase/MMP8 suggesting that macrophages and neutrophils were, at least in part, a source of cytokines and chemokines. These data support the idea that inflammatory cell-derived mediators contribute to the pathogenesis of SM induced skin damage. Modulating the infiltration of inflammatory cells and reducing the expression of inflammatory mediators in the skin may be an important strategy for mitigating SM-induced cutaneous injury.
Keywords: Dermal toxicity; Immuno-multiplex assays; Inflammatory cells infiltration; Inflammatory mediators; Sulfur mustard; Vesicants.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


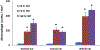

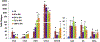
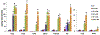
Similar articles
-
A type IV collagenase inhibitor, N-hydroxy-3-phenyl-2-(4-phenylbenzenesulfonamido) propanamide (BiPS), suppresses skin injury induced by sulfur mustard.Toxicol Appl Pharmacol. 2020 Aug 15;401:115078. doi: 10.1016/j.taap.2020.115078. Epub 2020 May 29. Toxicol Appl Pharmacol. 2020. PMID: 32479919 Free PMC article.
-
Time course of skin features and inflammatory biomarkers after liquid sulfur mustard exposure in SKH-1 hairless mice.Toxicol Lett. 2015 Jan 5;232(1):68-78. doi: 10.1016/j.toxlet.2014.09.022. Epub 2014 Sep 30. Toxicol Lett. 2015. PMID: 25275893
-
TRPA1 and CGRP antagonists counteract vesicant-induced skin injury and inflammation.Toxicol Lett. 2018 Sep 1;293:140-148. doi: 10.1016/j.toxlet.2018.03.007. Epub 2018 Mar 10. Toxicol Lett. 2018. PMID: 29535050 Free PMC article.
-
Tissue injury and repair following cutaneous exposure of mice to sulfur mustard.Ann N Y Acad Sci. 2016 Aug;1378(1):118-123. doi: 10.1111/nyas.13125. Epub 2016 Jul 2. Ann N Y Acad Sci. 2016. PMID: 27371823 Free PMC article. Review.
-
Acute and chronic effects of sulfur mustard on the skin: a comprehensive review.Cutan Ocul Toxicol. 2010 Dec;29(4):269-77. doi: 10.3109/15569527.2010.511367. Epub 2010 Sep 24. Cutan Ocul Toxicol. 2010. PMID: 20868209 Review.
Cited by
-
Attenuation of skin injury by a MARCO targeting PLGA nanoparticle.NPJ Regen Med. 2024 Dec 6;9(1):37. doi: 10.1038/s41536-024-00381-z. NPJ Regen Med. 2024. PMID: 39639015 Free PMC article.
-
Skin Models Used to Define Mechanisms of Action of Sulfur Mustard.Disaster Med Public Health Prep. 2023 Oct 18;17:e551. doi: 10.1017/dmp.2023.177. Disaster Med Public Health Prep. 2023. PMID: 37849329 Free PMC article. Review.
-
MicroRNA-mediated inflammation and coagulation effects in rats exposed to an inhaled analog of sulfur mustard.Ann N Y Acad Sci. 2020 Nov;1479(1):148-158. doi: 10.1111/nyas.14416. Epub 2020 Jun 29. Ann N Y Acad Sci. 2020. PMID: 32602122 Free PMC article.
-
A type IV collagenase inhibitor, N-hydroxy-3-phenyl-2-(4-phenylbenzenesulfonamido) propanamide (BiPS), suppresses skin injury induced by sulfur mustard.Toxicol Appl Pharmacol. 2020 Aug 15;401:115078. doi: 10.1016/j.taap.2020.115078. Epub 2020 May 29. Toxicol Appl Pharmacol. 2020. PMID: 32479919 Free PMC article.
-
Divinyl sulfone, an oxidative metabolite of sulfur mustard, induces caspase-independent pyroptosis in hepatocytes.Arch Toxicol. 2024 Mar;98(3):897-909. doi: 10.1007/s00204-023-03662-6. Epub 2024 Jan 3. Arch Toxicol. 2024. PMID: 38172301
References
-
- Arroyo CM, Schafer RJ, Kurt EM, Broomfield CA, Carmichael AJ, 2000. Response of normal human keratinocytes to sulfur mustard: cytokine release. J. Appl. Toxicol 20 (Suppl. 1), S63–S72. - PubMed
-
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M, 2008. Growth factors and cytokines in wound healing. Wound Repair Regen 16, 585–601. - PubMed
-
- Behm B, Babilas P, Landthaler M, Schreml S, 2012. Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol 26, 812–820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

