Brigatinib in Patients With Alectinib-Refractory ALK-Positive NSCLC
- PMID: 29935304
- PMCID: PMC6341982
- DOI: 10.1016/j.jtho.2018.06.005
Brigatinib in Patients With Alectinib-Refractory ALK-Positive NSCLC
Abstract
Introduction: The second-generation anaplastic lymphoma kinase (ALK) inhibitor alectinib recently showed superior efficacy compared to the first-generation ALK inhibitor crizotinib in advanced ALK-rearranged NSCLC, establishing alectinib as the new standard first-line therapy. Brigatinib, another second-generation ALK inhibitor, has shown substantial activity in patients with crizotinib-refractory ALK-positive NSCLC; however, its activity in the alectinib-refractory setting is unknown.
Methods: A multicenter, retrospective study was performed at three institutions. Patients were eligible if they had advanced, alectinib-refractory ALK-positive NSCLC and were treated with brigatinib. Medical records were reviewed to determine clinical outcomes.
Results: Twenty-two patients were eligible for this study. Confirmed objective responses to brigatinib were observed in 3 of 18 patients (17%) with measurable disease. Nine patients (50%) had stable disease on brigatinib. The median progression-free survival was 4.4 months (95% confidence interval [CI]: 1.8-5.6 months) with a median duration of treatment of 5.7 months (95% CI: 1.8-6.2 months). Among 9 patients in this study who underwent post-alectinib/pre-brigatinib biopsies, 5 had an ALK I1171X or V1180L resistance mutation; of these, 1 had a confirmed partial response and 3 had stable disease on brigatinib. One patient had an ALK G1202R mutation in a post-alectinib/pre-brigatinib biopsy, and had progressive disease as the best overall response to brigatinib.
Conclusions: Brigatinib has limited clinical activity in alectinib-refractory ALK-positive NSCLC. Additional studies are needed to establish biomarkers of response to brigatinib and to identify effective therapeutic options for alectinib-resistant ALK-positive NSCLC patients.
Keywords: ALK; Alectinib; Brigatinib; NSCLC; Resistance.
Copyright © 2018 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Figures
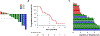


Comment in
-
How Should We Treat Alectinib-Refractory ALK-Positive Non-Small Cell Lung Cancer?J Thorac Oncol. 2018 Oct;13(10):1438-1440. doi: 10.1016/j.jtho.2018.07.009. J Thorac Oncol. 2018. PMID: 30244850 No abstract available.
References
-
- Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol 2011;12:175–180. - PubMed
-
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561–566. - PubMed
-
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167–2177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

