Using barcoded Zika virus to assess virus population structure in vitro and in Aedes aegypti mosquitoes
- PMID: 29935423
- PMCID: PMC6309320
- DOI: 10.1016/j.virol.2018.06.004
Using barcoded Zika virus to assess virus population structure in vitro and in Aedes aegypti mosquitoes
Abstract
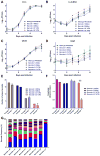
Arboviruses such as Zika virus (ZIKV, Flaviviridae; Flavivirus) must replicate in both mammalian and insect hosts possessing strong immune defenses. Accordingly, transmission between and replication within hosts involves genetic bottlenecks, during which viral population size and genetic diversity may be significantly reduced. To help quantify these bottlenecks and their effects, we constructed 4 "barcoded" ZIKV populations that theoretically contain thousands of barcodes each. After identifying the most diverse barcoded virus, we passaged this virus 3 times in 2 mammalian and mosquito cell lines and characterized the population using deep sequencing of the barcoded region of the genome. C6/36 maintain higher barcode diversity, even after 3 passages, than Vero. Additionally, field-caught mosquitoes exposed to the virus to assess bottlenecks in a natural host. A progressive reduction in barcode diversity occurred throughout systemic infection of these mosquitoes. Differences in bottlenecks during systemic spread were observed between different populations of Aedes aegypti.
Keywords: Arbovirus; Flavivirus; Mosquito-borne virus; Virus evolution; Zika virus.
Copyright © 2018. Published by Elsevier Inc.
Figures


References
-
- Duffy MR, Chen T-H, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. - PubMed
-
- Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, et al. Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Euro Surveill. 2014:19. Available: https://www.ncbi.nlm.nih.gov/pubmed/24626205. - PubMed
-
- Schuler-Faccini L, Ribeiro EM, Feitosa IML, Horovitz DDG, Cavalcanti DP, Pessoa A, et al. Possible Association Between Zika Virus Infection and Microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:59–62. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

