Alterations in cortical interneurons and cognitive function in schizophrenia
- PMID: 29936230
- PMCID: PMC6309598
- DOI: 10.1016/j.nbd.2018.06.020
Alterations in cortical interneurons and cognitive function in schizophrenia
Abstract
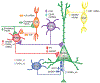
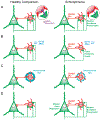
Certain clinical features of schizophrenia, such as working memory disturbances, appear to emerge from altered gamma oscillatory activity in the prefrontal cortex (PFC). Given the essential role of GABA neurotransmission in both working memory and gamma oscillations, understanding the cellular substrate for their disturbances in schizophrenia requires evidence from in vivo neuroimaging studies, which provide a means to link markers of GABA neurotransmission to gamma oscillations and working memory, and from postmortem studies, which provide insight into GABA neurotransmission at molecular and cellular levels of resolution. Here, we review findings from both types of studies which converge on the notions that 1) inhibitory GABA signaling in the PFC, especially between parvalbumin positive GABAergic basket cells and excitatory pyramidal cells, is required for gamma oscillatory activity and working memory function; and 2) disturbances in this signaling contribute to altered gamma oscillations and working memory in schizophrenia. Because the PFC is only one node in a distributed cortical network that mediates working memory, we also review evidence of GABA abnormalities in other cortical regions in schizophrenia.
Keywords: Cognition; GABA; Interneurons; Neuroimaging; Schizophrenia; Working memory.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
David A. Lewis currently receives investigator-initiated research support from Pfizer. In 2016–2018, he served as a consultant in the areas of target identification and validation and new compound development to Merck. Samuel Dienel declares no conflicts of interest.
Figures

References
-
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazoli A, Bunney WE, Jones EG, 1995. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in the prefrontal cortex of schizophrenics. Arch. Gen. Psychiatry 52, 258–266. - PubMed
-
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, DeFelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CCH, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R, 2008. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci 9, 557–568. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

