Mechanical transduction of cytoplasmic-to-transmembrane-domain movements in a hyperpolarization-activated cyclic nucleotide-gated cation channel
- PMID: 29936413
- PMCID: PMC6102142
- DOI: 10.1074/jbc.RA118.002139
Mechanical transduction of cytoplasmic-to-transmembrane-domain movements in a hyperpolarization-activated cyclic nucleotide-gated cation channel
Abstract
Hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channels play a critical role in the control of pacemaking in the heart and repetitive firing in neurons. In HCN channels, the intracellular cyclic nucleotide-binding domain (CNBD) is connected to the transmembrane portion of the channel (TMPC) through a helical domain, the C-linker. Although this domain is critical for mechanical signal transduction, the conformational dynamics in the C-linker that transmit the nucleotide-binding signal to the HCN channel pore are unknown. Here, we use linear response theory to analyze conformational changes in the C-linker of the human HCN1 protein, which couple cAMP binding in the CNBD with gating in the TMPC. By applying a force to the tip of the so-called "elbow" of the C-linker, the coarse-grained calculations recapitulate the same conformational changes triggered by cAMP binding in experimental studies. Furthermore, in our simulations, a displacement of the C-linker parallel to the membrane plane (i.e. horizontally) induced a rotational movement resulting in a distinct tilting of the transmembrane helices. This movement, in turn, increased the distance between the voltage-sensing S4 domain and the surrounding transmembrane domains and led to a widening of the intracellular channel gate. In conclusion, our computational approach, combined with experimental data, thus provides a more detailed understanding of how cAMP binding is mechanically coupled over long distances to promote voltage-dependent opening of HCN channels.
Keywords: HCN1 channel; anisotropic network model; cAMP dependent gating; computational biology; cyclic AMP (cAMP); linear response theory; potassium channel; protein conformation; protein dynamic.
© 2018 Gross et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
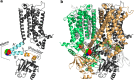

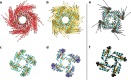





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

