The Inner Nuclear Membrane Is a Metabolically Active Territory that Generates Nuclear Lipid Droplets
- PMID: 29937227
- PMCID: PMC6371920
- DOI: 10.1016/j.cell.2018.05.047
The Inner Nuclear Membrane Is a Metabolically Active Territory that Generates Nuclear Lipid Droplets
Abstract
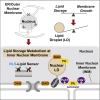
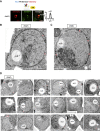
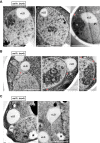
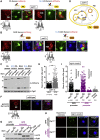
The inner nuclear membrane (INM) encases the genome and is fused with the outer nuclear membrane (ONM) to form the nuclear envelope. The ONM is contiguous with the endoplasmic reticulum (ER), the main site of phospholipid synthesis. In contrast to the ER and ONM, evidence for a metabolic activity of the INM has been lacking. Here, we show that the INM is an adaptable membrane territory capable of lipid metabolism. S. cerevisiae cells target enzymes to the INM that can promote lipid storage. Lipid storage involves the synthesis of nuclear lipid droplets from the INM and is characterized by lipid exchange through Seipin-dependent membrane bridges. We identify the genetic circuit for nuclear lipid droplet synthesis and a role of these organelles in regulating this circuit by sequestration of a transcription factor. Our findings suggest a link between INM metabolism and genome regulation and have potential relevance for human lipodystrophy.
Keywords: Lipin; Seipin; diacylglycerol; endoplasmic reticulum; inner nuclear membrane; lipid metabolism; lipid sensors; nuclear lipid droplets; phosphatidic acid; transcription factor.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures









Comment in
-
Lipid droplets from the inside.Nat Rev Mol Cell Biol. 2018 Aug;19(8):486-487. doi: 10.1038/s41580-018-0037-7. Nat Rev Mol Cell Biol. 2018. PMID: 29959402 No abstract available.
-
The Inner Nuclear Membrane Takes On Lipid Metabolism.Dev Cell. 2018 Nov 19;47(4):397-399. doi: 10.1016/j.devcel.2018.11.005. Dev Cell. 2018. PMID: 30458132
-
MUW researcher of the month.Wien Klin Wochenschr. 2019 Oct;131(19-20):532-533. doi: 10.1007/s00508-019-01563-2. Wien Klin Wochenschr. 2019. PMID: 31620880 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

