Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis
- PMID: 29937272
- PMCID: PMC6043361
- DOI: 10.1016/j.chom.2018.05.009
Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis
Abstract
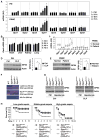
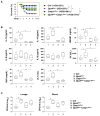
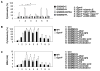
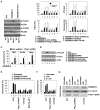
Sepsis is a life-threatening condition caused by pathogen infection and associated with pyroptosis. Pyroptosis occurs upon activation of proinflammatory caspases and their subsequent cleavage of gasdermin D (GSDMD), resulting in GSDMD N-terminal fragments that form membrane pores to induce cell lysis. Here, we show that antioxidant defense enzyme glutathione peroxidase 4 (GPX4) and its ability to decrease lipid peroxidation, negatively regulate macrophage pyroptosis, and septic lethality in mice. Conditional Gpx4 knockout in myeloid lineage cells increases lipid peroxidation-dependent caspase-11 activation and GSDMD cleavage. The resultant N-terminal GSDMD fragments then trigger macrophage pyroptotic cell death in a phospholipase C gamma 1 (PLCG1)-dependent fashion. Administration of the antioxidant vitamin E that reduces lipid peroxidation, chemical inhibition of PLCG1, or genetic Caspase-11 deletion or Gsdmd inactivation prevents polymicrobial sepsis in Gpx4-/- mice. Collectively, this study suggests that lipid peroxidation drives GSDMD-mediated pyroptosis and hence constitutes a potential therapeutic target for lethal infection.
Keywords: GPX4; GSDMD; caspase-11; ferroptosis; immunometabolism; inflammasome; lipid peroxidation; pyroptosis; sepsis.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest or financial interests.
Figures



Comment in
-
Lipid Peroxidation Adds Fuel to Pyr(optosis).Cell Host Microbe. 2018 Jul 11;24(1):8-9. doi: 10.1016/j.chom.2018.06.010. Cell Host Microbe. 2018. PMID: 30001526
References
-
- Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. - PubMed
-
- Brigelius-Flohe R, Maiorino M. Glutathione peroxidases. Biochimica et biophysica acta. 2013;1830:3289–3303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

