Layer I Interneurons Sharpen Sensory Maps during Neonatal Development
- PMID: 29937280
- PMCID: PMC6152945
- DOI: 10.1016/j.neuron.2018.06.002
Layer I Interneurons Sharpen Sensory Maps during Neonatal Development
Abstract
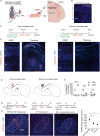
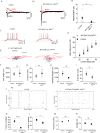
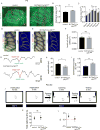
The neonatal mammal faces an array of sensory stimuli when diverse neuronal types have yet to form sensory maps. How these inputs interact with intrinsic neuronal activity to facilitate circuit assembly is not well understood. By using longitudinal calcium imaging in unanesthetized mouse pups, we show that layer I (LI) interneurons, delineated by co-expression of the 5HT3a serotonin receptor (5HT3aR) and reelin (Re), display spontaneous calcium transients with the highest degree of synchrony among cell types present in the superficial barrel cortex at postnatal day 6 (P6). 5HT3aR Re interneurons are activated by whisker stimulation during this period, and sensory deprivation induces decorrelation of their activity. Moreover, attenuation of thalamic inputs through knockdown of NMDA receptors (NMDARs) in these interneurons results in expansion of whisker responses, aberrant barrel map formation, and deficits in whisker-dependent behavior. These results indicate that recruitment of specific interneuron types during development is critical for adult somatosensory function. VIDEO ABSTRACT.
Keywords: barrel cortex; calcium imaging; development; interneuron; layer I; spontaneous activity; thalamocortical connectivity.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures





References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Allen Developing Mouse Brain Atlas. 2008 Available from: developingmouse.brain-map.org.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

