The Direct Involvement of Dark-Induced Tic55 Protein in Chlorophyll Catabolism and Its Indirect Role in the MYB108-NAC Signaling Pathway during Leaf Senescence in Arabidopsis thaliana
- PMID: 29937503
- PMCID: PMC6073118
- DOI: 10.3390/ijms19071854
The Direct Involvement of Dark-Induced Tic55 Protein in Chlorophyll Catabolism and Its Indirect Role in the MYB108-NAC Signaling Pathway during Leaf Senescence in Arabidopsis thaliana
Abstract
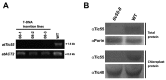
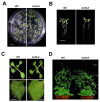
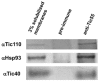
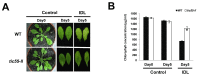
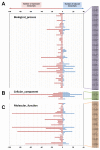
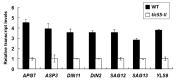
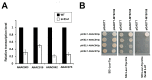
The chloroplast relies on proteins encoded in the nucleus, synthesized in the cytosol and subsequently transported into chloroplast through the protein complexes Toc and Tic (Translocon at the outer/inner membrane of chloroplasts). A Tic complex member, Tic55, contains a redox-related motif essential for protein import into chloroplasts in peas. However, Tic55 is not crucial for protein import in Arabidopsis. Here, a tic55-II-knockout mutant of Arabidopsis thaliana was characterized for Tic55 localization, its relationship with other translocon proteins, and its association with plant leaf senescence when compared to the wild type. Individually darkened leaves (IDLs) obtained through dark-induced leaf senescence were used to demonstrate chlorophyll breakdown and its relationship with plant senescence in the tic55-II-knockout mutant. The IDLs of the tic55-II-knockout mutant contained higher chlorophyll concentrations than those of the wild type. Our microarray analysis of IDLs during leaf senescence identified seven senescence-associated genes (SAGs) that were downregulated in the tic55-II-knockout mutant: ASP3, APG7, DIN2, DIN11, SAG12, SAG13, and YLS9. Real-time quantitative PCR confirmed the reliability of microarray analysis by showing the same expression patterns with those of the microarray data. Thus, Tic55 functions in dark-induced aging in A. thaliana by indirectly regulating downstream SAGs expression. In addition, the expression of four NAC genes, including ANAC003, ANAC010, ANAC042, and ANAC075 of IDL treated tic55-II-knockout mutant appeared to be downregulated. Yeast one hybrid assay revealed that only ANAC003 promoter region can be bound by MYB108, suggesting that a MYB-NAC regulatory network is involved in dark-stressed senescence.
Keywords: ANAC proteins; MYB108; Tic55 proteins of chloroplasts; dark-induced leaf senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

