Human P2Y11 Expression Level Affects Human P2X7 Receptor-Mediated Cell Death
- PMID: 29937766
- PMCID: PMC6002484
- DOI: 10.3389/fimmu.2018.01159
Human P2Y11 Expression Level Affects Human P2X7 Receptor-Mediated Cell Death
Abstract
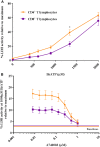
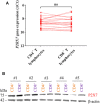
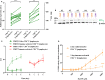
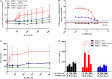
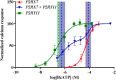
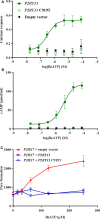
Adenosine triphosphate (ATP) is known to induce cell death in T lymphocytes at high extracellular concentrations. CD4+ and CD8+ T lymphocytes have a differential response to ATP, which in mice is due to differences in the P2X7 receptor expression levels. By contrast, we observed that the difference in human CD4+ and CD8+ T lymphocyte response toward the synthetic ATP-analog BzATP is not explained by a difference in human P2X7 receptor expression. Rather, the BzATP-induced human P2X7 receptor response in naïve and immune-activated lymphocyte subtypes correlated with the expression of another ATP-binding receptor: the human P2Y11 receptor. In a recombinant expression system, the coexpression of the human P2Y11 receptor counteracted BzATP-induced human P2X7 receptor-driven lactate dehydrogenase release (a marker of cell death) and pore formation independent of calcium signaling. A mutated non-signaling human P2Y11 receptor had a similar human P2X7 receptor-inhibitory effect on pore formation, thus demonstrating that the human P2X7 receptor interference was not caused by human P2Y11 receptor signaling. In conclusion, we demonstrate an important species difference in the ATP-mediated cell death between mice and human cells and show that in human T lymphocytes, the expression of the human P2Y11 receptor correlates with human P2X7 receptor-driven cell death following BzATP stimulation.
Keywords: A740003; Fluo-4; P2RX7; P2RY11; YO-PRO-1; cyclic adenosine monophosphate; purinergic.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

