Ototoxic effects and mechanisms of loop diuretics
- PMID: 29937824
- PMCID: PMC6002634
- DOI: 10.1016/j.joto.2016.10.001
Ototoxic effects and mechanisms of loop diuretics
Abstract
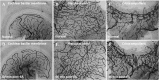
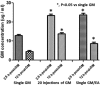
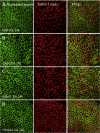
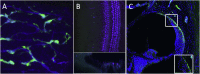
Over the past two decades considerable progress has been made in understanding the ototoxic effects and mechanisms underlying loop diuretics. As typical representative of loop diuretics ethacrynic acid or furosemide only induces temporary hearing loss, but rarely permanent deafness unless applied in severe acute or chronic renal failure or with other ototoxic drugs. Loop diuretic induce unique pathological changes in the cochlea such as formation of edematous spaces in the epithelium of the stria vascularis, which leads to rapid decrease of the endolymphatic potential and eventual loss of the cochlear microphonic potential, summating potential, and compound action potential. Loop diuretics interfere with strial adenylate cyclase and Na+/K+-ATPase and inhibit the Na-K-2Cl cotransporter in the stria vascularis, however recent reports indicate that one of the earliest effects in vivo is to abolish blood flow in the vessels supplying the lateral wall. Since ethacrynic acid does not damage the stria vascularis in vitro, the changes in Na+/K+-ATPase and Na-K-2Cl seen in vivo may be secondary effects results from strial ischemia and anoxia. Recent observations showing that renin is present in pericytes surrounding stria arterioles suggest that diuretics may induce local vasoconstriction by renin secretion and angiotensin formation. The tight junctions in the blood-cochlea barrier prevent toxic molecules and pathogens from entering cochlea, but when diuretics induce a transient ischemia, the barrier is temporarily disrupted allowing the entry of toxic chemicals or pathogens.
Keywords: Diuretics; Ischemia; Pericytes; Renin; Stria vascularis.
Figures




References
-
- Akiyoshi M. Effect of loop-diuretics on hair cells of the cochlea in Guinea pigs. Histological and histochemical study. Scand. Audiol. Suppl. 1981;(14 Suppl):185–199. - PubMed
-
- Arnold W., Nadol J.B., Jr., Weidauer H. Ultrastructural histopathology in a case of human ototoxicity due to loop diuretics. Acta Oto-Laryngol. 1981;91:399–414. - PubMed
-
- Bleich M., Greger R. Mechanism of action of diuretics. Kidney Int. Suppl. 1997;59:S11–S15. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

