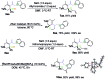
Rhodium-catalyzed asymmetric hydroamination and hydroindolation of keto-vinylidenecyclopropanes
- PMID: 29938038
- PMCID: PMC5994874
- DOI: 10.1039/c8sc01595c
Rhodium-catalyzed asymmetric hydroamination and hydroindolation of keto-vinylidenecyclopropanes
Abstract
We reported a highly regio- and enantioselective hydroamination and hydroindolation of keto-vinylidenecyclopropanes via cationic Rh(i) catalysis in this context. The combination of various secondary amines and indoles with keto-vinylidenecyclopropanes afforded the corresponding hydrofunctionalization products in good to excellent yields with outstanding ee values under mild conditions. A new TMM-Rh model complex was proposed, providing an atom economical Rh-π-allyl precursor at the same time. Moreover, the resulting products could easily be transformed into more complex polyheterocycles upon further synthetic manipulation.
Figures
References
-
-
For selected reviews on allylic substitutions, see
- Grange R. L., Clizbe E. A., Evans P. A. Synthesis. 2016;48:2911–2968.
- Oliver S., Evans P. A. Synthesis. 2013;45:3179–3198.
- Lu Z., Ma S.-M. Angew. Chem., Int. Ed. 2008;47:258–297. - PubMed
- Stoltz B. M., Mohr J. T. Chem. Asian J. 2007;2:1476–1491. - PMC - PubMed
- Miyabe H., Takemoto Y. Synlett. 2005;2005:1641–1655.
- Helmchen G. J. Org. Chem. 1999;576:203–214.
- Trost B. M., Vranken D. L. V. Chem. Rev. 1996;96:395–422. - PubMed
-
-
-
For selected reviews on the applications of allylic substitutions in organic synthesis, see
- Qu J.-P., Helmchen G. Acc. Chem. Res. 2017;50:2539–2555. - PubMed
- Huters A. D., Styduhar E. D., Garg N. K. Angew. Chem., Int. Ed. 2012;51:3758–3765. - PubMed
- Trost B. M., Crawley M. L. Chem. Rev. 2003;103:2921–2944. - PubMed
-
-
-
For selected reviews on allylic substitutions via allylic alcohols, see
- Butt N., Yang G.-Q., Zhang W.-B. Chem. Rec. 2016;16:2683–2692. - PubMed
- Butt N. A., Zhang W.-B. Chem. Soc. Rev. 2015;44:7929–7967. - PubMed
- Sundararaju B., Achard M., Bruneau C. Chem. Soc. Rev. 2012;41:4467–4483. - PubMed
- Bandini M. Angew. Chem., Int. Ed. 2011;50:994–995. - PubMed
- Szabó K. J. Synlett. 2006;2006:811–824.
- Tamaru Y. Eur. J. Org. Chem. 2005;2005:2647–2656.
- Muzart J. Tetrahedron. 2005;61:4179–4212.
-
-
-
For selected examples on allylic substitutions via allylic alcohols, see
- Jing J.-Y., Huo X.-H., Shen J.-F., Fu J.-K., Meng Q.-H., Zhang W.-B. Chem. Commun. 2017;53:5151–5154. - PubMed
- Huo X.-H., Yang G.-Q., Liu D.-L., Liu Y.-G., Gridnev I. D., Zhang W.-B. Angew. Chem., Int. Ed. 2014;53:6776–6780. - PubMed
- Shibuya R., Lin L., Nakahara Y., Mashima K., Ohshima T. Angew. Chem., Int. Ed. 2014;53:4377–4381. - PubMed
- Li Y.-X., Xuan Q.-Q., Liu L., Wang D., Chen Y.-J., Li C.-J. J. Am. Chem. Soc. 2013;135:12536–12539. - PubMed
- Das K., Shibuya R., Nakahara Y., Germain N., Ohshima T., Mashima K. Angew. Chem., Int. Ed. 2012;51:150–154. - PubMed
- Jiang G.-X., List B. Angew. Chem., Int. Ed. 2011;50:9471–9474. - PubMed
- Ohshima T., Miyamoto Y., Ipposhi J., Nakahara Y., Utsunomiya M., Mashima K. J. Am. Chem. Soc. 2009;131:14317–14328. - PubMed
- Defieber C., Ariger M. A., Moriel P., Carreira E. M. Angew. Chem., Int. Ed. 2007;46:3139–3143. - PubMed
- Ozawa F., Okamoto H., Kawagishi S., Yamamoto S., Minami T., Yoshifuji M. J. Am. Chem. Soc. 2002;124:10968–10969. - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous