Nanofabricated Ultraflexible Electrode Arrays for High-Density Intracortical Recording
- PMID: 29938162
- PMCID: PMC6010728
- DOI: 10.1002/advs.201700625
Nanofabricated Ultraflexible Electrode Arrays for High-Density Intracortical Recording
Abstract
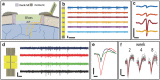
Understanding brain functions at the circuit level requires time-resolved simultaneous measurement of a large number of densely distributed neurons, which remains a great challenge for current neural technologies. In particular, penetrating neural electrodes allow for recording from individual neurons at high temporal resolution, but often have larger dimensions than the biological matrix, which induces significant damage to brain tissues and therefore precludes the high implant density that is necessary for mapping large neuronal populations with full coverage. Here, it is demonstrated that nanofabricated ultraflexible electrode arrays with cross-sectional areas as small as sub-10 µm2 can overcome this physical limitation. In a mouse model, it is shown that these electrodes record action potentials with high signal-to-noise ratio; their dense arrays allow spatial oversampling; and their multiprobe implantation allows for interprobe spacing at 60 µm without eliciting chronic neuronal degeneration. These results present the possibility of minimizing tissue displacement by implanted ultraflexible electrodes for scalable, high-density electrophysiological recording that is capable of complete neuronal circuitry mapping over chronic time scales.
Keywords: electron‐beam lithography; flexible neural electrodes; high‐density intracortical recording; in vivo extracellular recording; nanofabrication.
Figures




References
-
- Kotov N. A., Winter J. O., Clements I. P., Jan E., Timko B. P., Campidelli S., Pathak S., Mazzatenta A., Lieber C. M., Prato M., Bellamkonda R. V., Silva G. A., Kam N. W. S., Patolsky F., Ballerini L., Adv. Mater. 2009, 21, 3970.
-
- Nicolelis M. A., Ghazanfar A. A., Faggin B. M., Votaw S., Oliveira L. M., Neuron 1997, 18, 529. - PubMed
-
- Gray C. M., Maldonado P. E., Wilson M., McNaughton B., J. Neurosci. Methods 1995, 63, 43. - PubMed
-
- Wise K. D., Angell J. B., Starr A., IEEE Trans. Biomed. Eng. 1970, 17, 238. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
