Painful Terminal Neuroma Prevention by Capping PRGD/PDLLA Conduit in Rat Sciatic Nerves
- PMID: 29938170
- PMCID: PMC6010769
- DOI: 10.1002/advs.201700876
Painful Terminal Neuroma Prevention by Capping PRGD/PDLLA Conduit in Rat Sciatic Nerves
Abstract
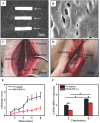
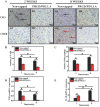
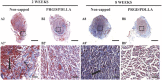
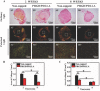
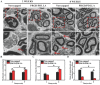
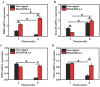
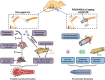
Neuroma formation after amputation as a long-term deficiency leads to spontaneous neuropathic pain that reduces quality of life of patients. To prevent neuroma formation, capping techniques are implemented as effective treatments. However, an ideal, biocompatible material covering the nerves is an unmet clinical need. In this study, biocompatible characteristics presented by the poly(D,L-lactic acid)/arginylglycylaspartic acid (RGD peptide) modification of poly{(lactic acid)-co- [(glycolic acid)-alt-(L-lysine)]} (PRGD/PDLLA) are evaluated as a nerve conduit. After being capped on the rat sciatic nerve stump in vivo, rodent behaviors and tissue structures are compared via autotomy scoring and histological analyses. The PRGD/PDLLA capped group gains lower autotomy score and improves the recovery, where inflammatory infiltrations and excessive collagen deposition are defeated. Transmission electron microscopy images of the regeneration of myelin sheath in both groups show that abnormal myelination is only present in the uncapped rats. Changes in related genes (MPZ, MBP, MAG, and Krox20) are monitored quantitative real-time polymerase chain reaction (qRT-PCR) for mechanism investigation. The PRGD/PDLLA capping conduits not only act as physical barriers to inhibit the invasion of inflammatory infiltration in the scar tissue but also provide a suitable microenvironment for promoting nerve repairing and avoiding neuroma formation during nerve recovery.
Keywords: inflammation; nerve conduits; neuroma prevention; painful scar neuropathy; scar deposition.
Figures
Similar articles
-
Surgical Approaches for Prevention of Neuroma at Time of Peripheral Nerve Injury.Front Surg. 2022 Jun 27;9:819608. doi: 10.3389/fsurg.2022.819608. eCollection 2022. Front Surg. 2022. PMID: 35832494 Free PMC article. Review.
-
Nerve Defect Treatment with a Capping Hydroxyethyl Cellulose/Soy Protein Isolate Sponge Conduit for Painful Neuroma Prevention.ACS Omega. 2023 Aug 17;8(34):30850-30858. doi: 10.1021/acsomega.3c00613. eCollection 2023 Aug 29. ACS Omega. 2023. PMID: 37663461 Free PMC article.
-
PDLLA/PRGD/β-TCP conduits build the neurotrophin-rich microenvironment suppressing the oxidative stress and promoting the sciatic nerve regeneration.J Biomed Mater Res A. 2014 Oct;102(10):3734-43. doi: 10.1002/jbm.a.35078. Epub 2014 Jan 27. J Biomed Mater Res A. 2014. PMID: 24408878
-
Nerve capping with a nerve conduit for the treatment of painful neuroma in the rat sciatic nerve.J Neurosurg. 2019 Feb 8;132(3):856-864. doi: 10.3171/2018.10.JNS182113. Print 2020 Mar 1. J Neurosurg. 2019. PMID: 30964248
-
Prevention and management of painful neuroma.Neurol Med Chir (Tokyo). 2006 Feb;46(2):62-7; discussion 67-8. doi: 10.2176/nmc.46.62. Neurol Med Chir (Tokyo). 2006. PMID: 16498214 Review.
Cited by
-
Highly Bioadaptable Hybrid Conduits with Spatially Bidirectional Structure for Precision Nerve Fiber Regeneration via Gene Therapy.Adv Sci (Weinh). 2024 May;11(19):e2309306. doi: 10.1002/advs.202309306. Epub 2024 Mar 14. Adv Sci (Weinh). 2024. PMID: 38483934 Free PMC article.
-
Surgical Approaches for Prevention of Neuroma at Time of Peripheral Nerve Injury.Front Surg. 2022 Jun 27;9:819608. doi: 10.3389/fsurg.2022.819608. eCollection 2022. Front Surg. 2022. PMID: 35832494 Free PMC article. Review.
-
Nerve Defect Treatment with a Capping Hydroxyethyl Cellulose/Soy Protein Isolate Sponge Conduit for Painful Neuroma Prevention.ACS Omega. 2023 Aug 17;8(34):30850-30858. doi: 10.1021/acsomega.3c00613. eCollection 2023 Aug 29. ACS Omega. 2023. PMID: 37663461 Free PMC article.
-
Macrophage Activation in the Dorsal Root Ganglion in Rats Developing Autotomy after Peripheral Nerve Injury.Int J Mol Sci. 2021 Nov 26;22(23):12801. doi: 10.3390/ijms222312801. Int J Mol Sci. 2021. PMID: 34884605 Free PMC article.
-
Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats.Theranostics. 2020 Jan 1;10(4):1590-1603. doi: 10.7150/thno.36272. eCollection 2020. Theranostics. 2020. PMID: 32042324 Free PMC article.
References
-
- Owings M. F., Kozak L. J., Vital Health Stat. 1998, 13, 139. - PubMed
-
- Ziegler‐Graham K., MacKenzie E. J., Ephraim P. L., Travison T. G., Brookmeyer R., Arch. Phys. Med. Rehabil. 2007, 89, 422. - PubMed
-
- a) Ahn H.‐S., Hwang J.‐Y., Kim M. S., Lee J.‐Y., Kim J.‐W., Kim H.‐S., Shin U. S., Knowles J. C., Kim H.‐W., Hyun J. K., Acta Biomater. 2015, 13, 324; - PubMed
- b) Mirsky R., Woodhoo A., Parkinson D. B., Arthur‐Farraj P., Bhaskaran A., Jessen K. R., J. Peripher. Nerv. Syst. 2008, 13, 122; - PubMed
- c) Menorca R. M. G., Fussell T. S., Elfar J. C., Hand Clin. 2013, 29, 317. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
