Locomotor- and Reward-Enhancing Effects of Cocaine Are Differentially Regulated by Chemogenetic Stimulation of Gi-Signaling in Dopaminergic Neurons
- PMID: 29938215
- PMCID: PMC6011418
- DOI: 10.1523/ENEURO.0345-17.2018
Locomotor- and Reward-Enhancing Effects of Cocaine Are Differentially Regulated by Chemogenetic Stimulation of Gi-Signaling in Dopaminergic Neurons
Abstract
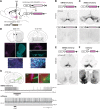
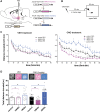
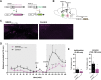
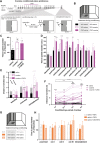
Dopamine plays a key role in the cellular and behavioral responses to drugs of abuse, but the implication of metabotropic regulatory input to dopaminergic neurons on acute drug effects and subsequent drug-related behavior remains unclear. Here, we used chemogenetics [Designer Receptors Exclusively Activated by Designer Drugs (DREADDs)] to modulate dopamine signaling and activity before cocaine administration in mice. We show that chemogenetic inhibition of dopaminergic ventral tegmental area (VTA) neurons differentially affects locomotor and reward-related behavioral responses to cocaine. Stimulation of Gi-coupled DREADD (hM4Di) expressed in dopaminergic VTA neurons persistently reduced the locomotor response to repeated cocaine injections. An attenuated locomotor response was seen even when a dual-viral vector approach was used to restrict hM4Di expression to dopaminergic VTA neurons projecting to the nucleus accumbens. Surprisingly, despite the attenuated locomotor response, hM4Di-mediated inhibition of dopaminergic VTA neurons did not prevent cocaine sensitization, and the inhibitory effect of hM4Di-mediated inhibition was eliminated after withdrawal. In the conditioned place-preference paradigm, hM4Di-mediated inhibition did not affect cocaine-induced place preference; however, the extinction period was extended. Also, hM4Di-mediated inhibition had no effect on preference for a sugar-based reward over water but impaired motivation to work for the same reward in a touchscreen-based motivational assay. In addition, to support that VTA dopaminergic neurons operate as regulators of reward motivation toward both sugar and cocaine, our data suggest that repeated cocaine exposure leads to adaptations in the VTA that surmount the ability of Gi-signaling to suppress and regulate VTA dopaminergic neuronal activity.
Keywords: G protein–coupled receptors; addiction; chemogenetics; cocaine; dopamine; mouse behavior.
Figures




Similar articles
-
Chemogenetic Manipulations of Ventral Tegmental Area Dopamine Neurons Reveal Multifaceted Roles in Cocaine Abuse.J Neurosci. 2019 Jan 16;39(3):503-518. doi: 10.1523/JNEUROSCI.0537-18.2018. Epub 2018 Nov 16. J Neurosci. 2019. PMID: 30446532 Free PMC article.
-
Re-examining the role of ventral tegmental area dopaminergic neurons in motor activity and reinforcement by chemogenetic and optogenetic manipulation in mice.Metab Brain Dis. 2019 Oct;34(5):1421-1430. doi: 10.1007/s11011-019-00442-z. Epub 2019 Jul 16. Metab Brain Dis. 2019. PMID: 31313126
-
G protein-coupled receptor signaling in VTA dopaminergic neurons bidirectionally regulates the acute locomotor response to amphetamine but does not affect behavioral sensitization.Neuropharmacology. 2019 Dec 15;161:107663. doi: 10.1016/j.neuropharm.2019.06.002. Epub 2019 Jun 4. Neuropharmacology. 2019. PMID: 31173760
-
Dopamine neurons gate the intersection of cocaine use, decision making, and impulsivity.Addict Biol. 2021 Nov;26(6):e13022. doi: 10.1111/adb.13022. Epub 2021 Feb 8. Addict Biol. 2021. PMID: 33559379 Review.
-
Ventral tegmental area dopamine revisited: effects of acute and repeated stress.Psychopharmacology (Berl). 2016 Jan;233(2):163-86. doi: 10.1007/s00213-015-4151-3. Epub 2015 Dec 17. Psychopharmacology (Berl). 2016. PMID: 26676983 Free PMC article. Review.
Cited by
-
Behavioral encoding across timescales by region-specific dopamine dynamics.Proc Natl Acad Sci U S A. 2023 Feb 14;120(7):e2215230120. doi: 10.1073/pnas.2215230120. Epub 2023 Feb 7. Proc Natl Acad Sci U S A. 2023. PMID: 36749722 Free PMC article.
-
Comparison of three DREADD agonists acting on Gq-DREADDs in the ventral tegmental area to alter locomotor activity in tyrosine hydroxylase:Cre male and female rats.Behav Brain Res. 2023 Oct 18;455:114674. doi: 10.1016/j.bbr.2023.114674. Epub 2023 Sep 16. Behav Brain Res. 2023. PMID: 37722510 Free PMC article.
-
Medial orbitofrontal, prefrontal and amygdalar circuits support dissociable component processes of risk/reward decision making.J Neurosci. 2025 Mar 11;45(16):e2147242025. doi: 10.1523/JNEUROSCI.2147-24.2025. Online ahead of print. J Neurosci. 2025. PMID: 40068869
-
Altered baseline and amphetamine-mediated behavioral profiles in dopamine transporter Cre (DAT-Ires-Cre) mice compared to tyrosine hydroxylase Cre (TH-Cre) mice.Psychopharmacology (Berl). 2020 Dec;237(12):3553-3568. doi: 10.1007/s00213-020-05635-4. Epub 2020 Aug 10. Psychopharmacology (Berl). 2020. PMID: 32778904 Free PMC article.
-
Prenatal and postnatal alcohol exposure increases vulnerability to cocaine addiction in adult mice.Br J Pharmacol. 2020 Mar;177(5):1090-1105. doi: 10.1111/bph.14901. Epub 2020 Jan 23. Br J Pharmacol. 2020. PMID: 31705540 Free PMC article.
References
-
- Apuschkin M, Stilling S, Rahbek-Clemmensen T, Sørensen G, Fortin G, Herborg Hansen F, Eriksen J, Trudeau L-E, Egerod K, Gether U, Rickhag M (2015) A novel dopamine transporter transgenic mouse line for identification and purification of midbrain dopaminergic neurons reveals midbrain heterogeneity. Eur J Neurosci 42:2438–2454. 10.1111/ejn.13046 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
