dSTIM- and Ral/Exocyst-Mediated Synaptic Release from Pupal Dopaminergic Neurons Sustains Drosophila Flight
- PMID: 29938216
- PMCID: PMC6011419
- DOI: 10.1523/ENEURO.0455-17.2018
dSTIM- and Ral/Exocyst-Mediated Synaptic Release from Pupal Dopaminergic Neurons Sustains Drosophila Flight
Abstract
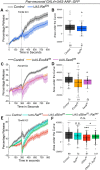
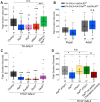
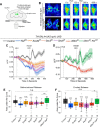
Manifestation of appropriate behavior in adult animals requires developmental mechanisms that help in the formation of correctly wired neural circuits. Flight circuit development in Drosophila requires store-operated calcium entry (SOCE) through the STIM/Orai pathway. SOCE-associated flight deficits in adult Drosophila derive extensively from regulation of gene expression in pupal neurons, and one such SOCE-regulated gene encodes the small GTPase Ral. The cellular mechanism by which Ral helps in maturation of the flight circuit was not understood. Here, we show that knockdown of components of a Ral effector, the exocyst complex, in pupal neurons also leads to reduced flight bout durations, and this phenotype derives primarily from dopaminergic neurons. Importantly, synaptic release from pupal dopaminergic neurons is abrogated upon knockdown of dSTIM, Ral, or exocyst components. Ral overexpression restores the diminished synaptic release of dStim knockdown neurons as well as flight deficits associated with dSTIM knockdown in dopaminergic neurons. These results identify Ral-mediated vesicular release as an effector mechanism of neuronal SOCE in pupal dopaminergic neurons with functional consequences on flight behavior.
Keywords: Exo84; Neural Circuit; SOCE; Synaptic Maturation.
Figures


Similar articles
-
A pupal transcriptomic screen identifies Ral as a target of store-operated calcium entry in Drosophila neurons.Sci Rep. 2017 Feb 14;7:42586. doi: 10.1038/srep42586. Sci Rep. 2017. PMID: 28195208 Free PMC article.
-
Store-Operated Calcium Entry through Orai Is Required for Transcriptional Maturation of the Flight Circuit in Drosophila.J Neurosci. 2015 Oct 7;35(40):13784-99. doi: 10.1523/JNEUROSCI.1680-15.2015. J Neurosci. 2015. PMID: 26446229 Free PMC article.
-
Ral function in muscle is required for flight maintenance in Drosophila.Small GTPases. 2020 May;11(3):174-179. doi: 10.1080/21541248.2017.1367456. Epub 2017 Dec 28. Small GTPases. 2020. PMID: 29284321 Free PMC article.
-
Ral: mediator of membrane trafficking.Int J Biochem Cell Biol. 2006;38(11):1841-7. doi: 10.1016/j.biocel.2006.04.006. Epub 2006 May 9. Int J Biochem Cell Biol. 2006. PMID: 16781882 Review.
-
The STIM-Orai Pathway: STIM-Orai Structures: Isolated and in Complex.Adv Exp Med Biol. 2017;993:15-38. doi: 10.1007/978-3-319-57732-6_2. Adv Exp Med Biol. 2017. PMID: 28900907 Review.
Cited by
-
ER-Ca2+ sensor STIM regulates neuropeptides required for development under nutrient restriction in Drosophila.PLoS One. 2019 Jul 11;14(7):e0219719. doi: 10.1371/journal.pone.0219719. eCollection 2019. PLoS One. 2019. PMID: 31295329 Free PMC article.
-
Modulation of flight and feeding behaviours requires presynaptic IP3Rs in dopaminergic neurons.Elife. 2020 Nov 6;9:e62297. doi: 10.7554/eLife.62297. Elife. 2020. PMID: 33155978 Free PMC article.
-
Allatostatin-C/AstC-R2 Is a Novel Pathway to Modulate the Circadian Activity Pattern in Drosophila.Curr Biol. 2019 Jan 7;29(1):13-22.e3. doi: 10.1016/j.cub.2018.11.005. Epub 2018 Dec 13. Curr Biol. 2019. PMID: 30554904 Free PMC article.
-
Identification and Analysis of MicroRNAs Associated with Wing Polyphenism in the Brown Planthopper, Nilaparvata lugens.Int J Mol Sci. 2020 Dec 21;21(24):9754. doi: 10.3390/ijms21249754. Int J Mol Sci. 2020. PMID: 33371331 Free PMC article.
References
-
- Agrawal N, Venkiteswaran G, Sadaf S, Padmanabhan N, Banerjee S, Hasan G (2010) Inositol 1,4,5-trisphosphate receptor and dSTIM function in Drosophila insulin-producing neurons regulates systemic intracellular calcium homeostasis and flight. J Neurosci 30:1301–1313. 10.1523/JNEUROSCI.3668-09.2010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
