Overview of Japanese encephalitis disease and its prevention. Focus on IC51 vaccine (IXIARO®)
- PMID: 29938245
- PMCID: PMC6009073
- DOI: 10.15167/2421-4248/jpmh2018.59.1.962
Overview of Japanese encephalitis disease and its prevention. Focus on IC51 vaccine (IXIARO®)
Abstract
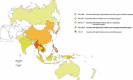
Japanese encephalitis (JE) is a vector-borne disease caused by the Japanese encephalitis virus (JEV). JEV is transmitted by mosquitoes to a wide range of vertebrate hosts, including birds and mammals. Domestic animals, especially pigs, are generally implicated as reservoirs of the virus, while humans are not part of the natural transmission cycle and cannot pass the virus to other hosts. Although JEV infection is very common in endemic areas (many countries in Asia), less than 1% of people affected develop clinical disease, and severe disease affects about 1 case per 250 JEV infections. Although rare, severe disease can be devastating; among the 30,000-50,000 global cases per year, approximately 20-30% of patients die and 30-50% of survivors develop significant neurological sequelae. JE is a significant public health problem for residents in endemic areas and may constitute a substantial risk for travelers to these areas. The epidemiology of JE and its risk to travelers have changed, and continue to evolve. The rapid economic growth of Asian countries has led to a surge in both inbound and outbound travel, making Asia the second most-visited region in the world after Europe, with 279 million international travelers in 2015. The top destination is China, followed by Thailand, Hong Kong, Malaysia and Japan, and the number of travelers is forecast to reach 535 million by 2030 (+ 4.9% per year). Because of the lack of treatment and the infeasibility of eliminating the vector, vaccination is recognized as the most efficacious means of preventing JE. The IC51 vaccine (IXIARO®) is a purified, inactivated, whole virus vaccine against JE. It is safe, well tolerated, efficacious and can be administered to children, adults and the elderly. The vaccination schedule involves administering 2 doses four weeks apart. For adults, a rapid schedule (0-7 days) is available, which could greatly enhance the feasibility of its use. Healthcare workers should inform both short- and long-term travelers of the risk of JE in each period of the year and recommend vaccination. Indeed, it has been shown that short-term travelers are also at risk, not only in rural environments, but also in cities and coastal towns, especially in tourist localities where excursions to country areas are organized.
Keywords: IC51 vaccine (IXIARO®); Japanese encephalitis disease; Japanese encephalitis virus; Travel medicine.
Figures
References
-
- Hills S, Rabe I, Fischer M. Japanese Encephalitis. CDC Yellow Book, 2016. Available at: https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related.... [Accessed on 10 October 2017].
-
- WHO Report. Japanese encephalitis vaccines: WHO position paper, February 2015- Recommendations. Vaccines 2016;34:302-3. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources