Crawling wounded: molecular genetic insights into wound healing from Drosophila larvae
- PMID: 29938760
- PMCID: PMC6352908
- DOI: 10.1387/ijdb.180085mg
Crawling wounded: molecular genetic insights into wound healing from Drosophila larvae
Abstract
For animals, injury is inevitable. Because of this, organisms possess efficient wound healing mechanisms that can repair damaged tissues. However, the molecular and genetic mechanisms by which epidermal repair is accomplished remain poorly defined. Drosophila has become a valuable model to study epidermal wound healing because of the comprehensive genetic toolkit available in this organism and the similarities of wound healing processes between Drosophila and vertebrates. Other reviews in this Special Issue cover wound healing assays and pathways in Drosophila embryos, pupae and adults, as well as regenerative processes that occur in tissues such as imaginal discs and the gut. In this review, we will focus on the molecular/genetic control of wound-induced cellular processes such as inflammation, cell migration and epithelial cell-cell fusion in Drosophila larvae. We will give a brief overview of the three wounding assays, pinch, puncture, and laser ablation, and the cellular responses that ensue following wounding. We will highlight the actin regulators, signaling pathways and transcriptional mediators found so far to be involved in larval epidermal wound closure and what is known about how they act. We will also discuss wound-induced epidermal cell-cell fusion and possible directions for future research in this exciting system.
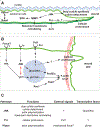
Figures


Similar articles
-
Drosophila as a model of wound healing and tissue regeneration in vertebrates.Dev Dyn. 2011 Nov;240(11):2379-404. doi: 10.1002/dvdy.22753. Epub 2011 Sep 26. Dev Dyn. 2011. PMID: 21953647 Review.
-
JNK signaling pathway is required for efficient wound healing in Drosophila.Dev Biol. 2002 Jan 1;241(1):145-56. doi: 10.1006/dbio.2001.0502. Dev Biol. 2002. PMID: 11784101
-
Active cop, passive cop: developmental stage-specific modes of wound-induced blood cell recruitment in Drosophila.Fly (Austin). 2008 Nov-Dec;2(6):303-5. doi: 10.4161/fly.7395. Epub 2008 Nov 13. Fly (Austin). 2008. PMID: 19077535
-
Tissue repair and regeneration in Drosophila imaginal discs.Dev Growth Differ. 2011 Feb;53(2):177-85. doi: 10.1111/j.1440-169X.2010.01247.x. Dev Growth Differ. 2011. PMID: 21338344 Review.
-
Transcriptional regulation of Profilin during wound closure in Drosophila larvae.J Cell Sci. 2012 Dec 1;125(Pt 23):5667-76. doi: 10.1242/jcs.107490. Epub 2012 Sep 12. J Cell Sci. 2012. PMID: 22976306 Free PMC article.
Cited by
-
Deep learning reveals a damage signalling hierarchy that coordinates different cell behaviours driving wound re-epithelialisation.Development. 2024 Sep 15;151(18):dev202943. doi: 10.1242/dev.202943. Epub 2024 Sep 24. Development. 2024. PMID: 39177163 Free PMC article.
-
Wound Healing in Butterfly Pupal Wing Tissues: Real-Time In Vivo Imaging of Long-Range Cell Migration, Cluster Formation, and Calcium Oscillations.Insects. 2025 Jan 27;16(2):124. doi: 10.3390/insects16020124. Insects. 2025. PMID: 40003754 Free PMC article.
-
Interplay between integrins and PI4P5K Sktl is crucial for cell polarization and reepithelialisation during Drosophila wound healing.Sci Rep. 2019 Nov 8;9(1):16331. doi: 10.1038/s41598-019-52743-z. Sci Rep. 2019. PMID: 31704968 Free PMC article.
-
cdc37 is essential for JNK pathway activation and wound closure in Drosophila.Mol Biol Cell. 2019 Oct 1;30(21):2651-2658. doi: 10.1091/mbc.E18-12-0822. Epub 2019 Sep 4. Mol Biol Cell. 2019. PMID: 31483695 Free PMC article.
-
Model systems for regeneration: Drosophila.Development. 2020 Apr 6;147(7):dev173781. doi: 10.1242/dev.173781. Development. 2020. PMID: 32253254 Free PMC article. Review.
References
-
- AHRINGER J (2000). NuRD and SIN3: Histone deacetylase complexes in development. Trends Genet 16: 351–356. - PubMed
-
- ARAGONA M, PANCIERA T, MANFRIN A, GIULITTI S, MICHIELIN F, ELVASSORE N, DUPONT S, PICCOLO S (2013). A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154: 1047–1059. - PubMed
-
- AYER DE (1999). Histone deacetylases: Transcriptional repression with SINers and NuRDs. Trends Cell Biol 9: 193–198. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

