Homeostasis, regeneration and tumour formation in the mammalian epidermis
- PMID: 29938768
- PMCID: PMC6103439
- DOI: 10.1387/ijdb.170341fw
Homeostasis, regeneration and tumour formation in the mammalian epidermis
Abstract
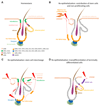
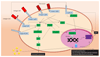
The epidermis is the outer covering of the skin and provides a protective interface between the body and the environment. It is well established that the epidermis is maintained by stem cells that self-renew and generate differentiated cells. In this review, we discuss how recent technological advances, including single cell transcriptomics and in vivo imaging, have provided new insights into the nature and plasticity of the stem cell compartment and the differing roles of stem cells in homeostasis, wound repair and cancer.
Figures



References
-
- Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer. 2012;12:170–180. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

