High V-PPase activity is beneficial under high salt loads, but detrimental without salinity
- PMID: 29938800
- PMCID: PMC6099232
- DOI: 10.1111/nph.15280
High V-PPase activity is beneficial under high salt loads, but detrimental without salinity
Abstract
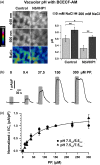
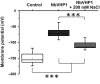
The membrane-bound proton-pumping pyrophosphatase (V-PPase), together with the V-type H+ -ATPase, generates the proton motive force that drives vacuolar membrane solute transport. Transgenic plants constitutively overexpressing V-PPases were shown to have improved salinity tolerance, but the relative impact of increasing PPi hydrolysis and proton-pumping functions has yet to be dissected. For a better understanding of the molecular processes underlying V-PPase-dependent salt tolerance, we transiently overexpressed the pyrophosphate-driven proton pump (NbVHP) in Nicotiana benthamiana leaves and studied its functional properties in relation to salt treatment by primarily using patch-clamp, impalement electrodes and pH imaging. NbVHP overexpression led to higher vacuolar proton currents and vacuolar acidification. After 3 d in salt-untreated conditions, V-PPase-overexpressing leaves showed a drop in photosynthetic capacity, plasma membrane depolarization and eventual leaf necrosis. Salt, however, rescued NbVHP-hyperactive cells from cell death. Furthermore, a salt-induced rise in V-PPase but not of V-ATPase pump currents was detected in nontransformed plants. The results indicate that under normal growth conditions, plants need to regulate the V-PPase pump activity to avoid hyperactivity and its negative feedback on cell viability. Nonetheless, V-PPase proton pump function becomes increasingly important under salt stress for generating the pH gradient necessary for vacuolar proton-coupled Na+ sequestration.
Keywords: cell death; plasma membrane voltage; proton pump currents; salt; vacuolar pH; vacuolar proton-ATPase (V-ATPase); vacuolar proton-pyrophosphatase (V-PPase).
© 2018 The Authors. New Phytologist © 2018 New Phytologist Trust.
Figures



References
-
- Apse MP, Aharon GS, Snedden WA, Blumwald E. 1999. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285: 1256–1258. - PubMed
-
- Apse MP, Sottosanto JB, Blumwald E. 2003. Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T‐DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant Journal 36: 229–239. - PubMed
-
- Beyhl D, Hörtensteiner S, Martinoia E, Farmer EE, Fromm J, Marten I, Hedrich R. 2009. The fou2 mutation in the major vacuolar cation channel TPC1 confers tolerance to inhibitory luminal calcium. Plant Journal 58: 715–723. - PubMed
-
- Choura M, Rebai A. 2005. Identification and characterization of new members of vacuolar H+‐pyrophosphatase family from Oryza sativa genome. Russian Journal of Plant Physiology 52: 821–825.
-
- Colombo R, Cerana R. 1993. Effect of temperature on plasma membrane and tonoplast ion channels in Arabidopsis thaliana . Physiologia Plantarum 87: 118–124.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

